路易斯酸催化系统对异丁烯聚合的调谐:双人组合比单人组合更好吗?
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了用于合成中分子量聚异丁烯(MPIB)的 FeCl3-苯乙醚/TiCl4-H2O 催化体系。双路易斯酸体系对催化效率有显著的协同作用。与 FeCl3-苯乙醚体系相比,双路易斯酸体系实现了更高的单体转化率。值得注意的是,双路易斯酸体系在 10 分钟内获得了平均分子量高达 18,500 g mol-1 的 PIB,转化率为 93%。DFT 计算显示,FeCl3-苯乙醚/TiCl4-H2O 复合物具有二聚 FeCl3/TiCl4 几何结构,其中 H2O 同时与 Fe 中心和 Ti 中心结合。在 FeCl3-苯乙醚/TiCl4-H2O 体系中,启动反应的最合理机制是质子从 H2O 分子转移到附近的异丁烯分子,与 FeCl3-苯乙醚体系启动的反应相比,这种机制在能障方面更有利。本文章由计算机程序翻译,如有差异,请以英文原文为准。
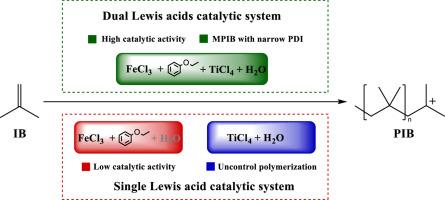
Tuning of isobutylene polymerization by Lewis acid catalytic Systems: Is a duo better than one?
The FeCl3·phenetole/TiCl4·H2O catalytic system was investigated for the synthesis of medium molecular weight polyisobutylene (MPIB). The dual Lewis acid system showed a remarkable synergistic effect on the catalytic efficiency. The much higher monomer conversion was achieved with the dual Lewis acid system than that with the FeCl3·phenetole system. Notably, the PIB with number average molecular weight up to 18,500 g mol-1 was obtained with 93 % conversion in 10 min by the dual Lewis acid system. DFT calculations revealed a dimeric FeCl3/TiCl4 geometry for the FeCl3·phenetole/TiCl4·H2O complex, in which H2O simultaneously binds to the Fe center and the Ti center. The most plausible mechanism for the initiation reaction follows a proton transfer from the H2O molecule to a nearby isobutylene molecule in the FeCl3·phenetole/TiCl4·H2O system, which is favored in terms of energy barrier with comparison to the reaction initiated by the FeCl3·phenetole system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


