基于苯并二噻唑和罗丹宁的有机太阳能电池低能级小分子供体
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
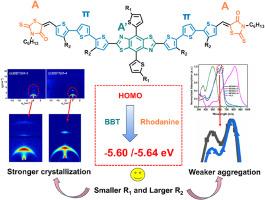
目前,用于有机太阳能电池(OSC)的高性能小分子供体几乎全部由电子供体核心苯并二噻吩(BDT)组成,这导致材料缺乏多样性和进一步改进。本文利用电子汲取基团苯并二噻唑(BBT)核心和罗丹宁末端基团构建了 A-π-A′-π-A 共轭结构,并合成了两种小分子供体 BBTSM-3 和 BBTSM-4。在BBT和罗丹宁的双重退电子作用下,BBTSM-3和BBTSM-4的HOMO能级达到了极低的水平(-5.60 eV和-5.64 eV),但仍能与受体Y6很好地匹配。此外,通过合理的侧链调节,BBTSM-3 同时表现出更强的结晶性和更弱的聚集性。这为小分子供体中电子撤回核的使用和侧链的调节提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Benzobisthiazole and rhodanine based low energy level small molecule donors for organic solar cells
At present, high-performance small molecule donors for organic solar cells (OSCs) are almost entirely composed of the electron donating core benzodithiophene (BDT), which has resulted in a lack of material diversity and further improvement. In this article, we constructed an A-π-A′-π-A conjugated structure using electron withdrawing group benzobisthiazole (BBT) core and rhodanine end group, and synthesized two small molecule donors BBTSM-3 and BBTSM-4. Under the dual electron withdrawing effects of BBT and rhodanine, the HOMO energy levels of BBTSM-3 and BBTSM-4 reached extremely low levels (−5.60 eV and −5.64 eV), but still matched well with the acceptor Y6. In addition, through reasonable side chain regulation, BBTSM-3 exhibited stronger crystallinity and weaker aggregation at the same time. This provides a reference point for the use of the electron withdrawing core and the regulation of side chains among small molecule donors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


