基于 ESIPT 机制的高选择性锌离子比率荧光传感器
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
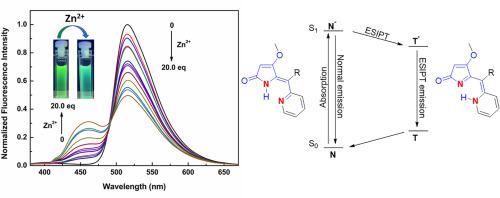
锌对人类健康至关重要,这一事实激发了人们对开发高效荧光探针检测锌的兴趣。然而,传统方法面临着环境干扰和选择性有限等问题。因此,研究重点放在了比率测量传感和利用激发态分子内质子转移(ESIPT)效应上。四元酸及其 4-甲氧基类似物是来自各种生物的许多天然产物中的重要结构基团,包括那些能与锌等金属离子结合的天然产物。本研究提出了一种新的锌比率荧光传感器,它利用 ESIPT 实现了高选择性和高效性,并结合了四元酸和吡啶的亚结构。我们的探针在极性非沸腾溶剂中被激发后会在 515 纳米处显示出强发射带,在乙腈中被激发后会在 360 纳米处显示出对 Zn2+ 的选择性比率反应,并在 460 纳米处显示出明显的蓝移发射最大值。随之而来的是颜色从绿色变为绿松石色,在紫外灯下肉眼可见。核磁共振研究进一步证实了这些化合物与 Zn2+ 离子的结合,还研究了常见阴离子和 pH 值变化的影响。我们的结构总体上比较简单,同时具有独特的性质和结构修饰的可能性,因此对进一步的研究很有吸引力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A highly selective ESIPT-mechanism-based, ratiometric fluorescent sensor for zinc ions
Zinc is crucial for human health, a fact that has prompted interest in developing efficient fluorescent probes for its detection. Traditional approaches, however, face issues such as environmental interference and limited selectivity. This has led to research focusing on ratiometric sensing and making use of the excited-state intramolecular proton transfer (ESIPT) effect. Tetramic acid and its 4-methoxy analogue are significant structural motifs in many natural products from various organisms, including those which can bind metal ions, such as zinc. This study presents a new ratiometric fluorescent sensor for zinc that leverages ESIPT for high selectivity and efficacy, and combines tetramic acid and pyridine substructures. Our probe exhibits a strong emission band at 515 nm upon excitation in polar aprotic solvents, and upon excitation at 360 nm a selective ratiometric response to Zn2+ in acetonitrile, with distinct, blue-shifted emission maximum at 460 nm. This is accompanied by a color change from green to turquoise, which is visible to the unaided eye under a UV lamp. Our sensor shows excellent sensitivity, which is confirmed by a low detection limit of 1.26 × 10−6 M. The binding of the compounds to Zn2+ ions was further confirmed in an NMR study, and the effects of common anions and change in pH were also examined. The overall simplicity of our structure, alongside its unique properties and open possibilities for structural modifications, makes it attractive for further research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


