非病毒性肝病的肝细胞癌风险评分:系统回顾和荟萃分析
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
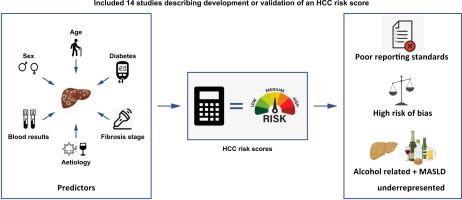
背景& 目的肝细胞癌(HCC)风险预测模型可为肝硬化患者的HCC监测提供更加个性化的方法。本系统性综述旨在总结HCC预测模型在非病毒性慢性肝病患者中的表现。方法该研究在PROSPERO(ID:CRD42022370078)进行了前瞻性注册,并按照PRISMA指南进行了报告。使用经过验证的预测模型研究检索过滤器对 MEDLINE 和 Embase 数据库进行检索。两名审稿人独立评估研究的纳入情况和偏倚风险。确定了评估特定时间点 HCC 风险的模型性能(区分度和校准)的衡量标准。对报告同一模型性能的研究子集进行了随机效应荟萃分析。经审查后,共纳入了 14 项研究,共有 94,014 名参与者;45% 的患者患有病毒性肝炎,27% 的患者患有 ALD(酒精相关性肝病),19% 的患者患有 MASLD(代谢功能障碍相关性脂肪肝)。随访时间为 15.1-138 个月。只有一个模型采用了竞争风险方法。年龄(7 个模型)和性别(6 个模型)是最常见的预测因素。模型区分度(AUROC 或 c 统计量)介于 0.61-0.947 之间。只有 "aMAP "评分(年龄、男性性别、白蛋白、胆红素和血小板)在定量分析中得到了充分的外部验证,其汇总 c 统计量为 0.81(95% CI 0.80-0.83)。14 项研究中只有 9 项报告了校准结果。结论有关非病毒性慢性肝病 HCC 风险预测的研究报告很少,偏倚风险很高,而且没有考虑竞争风险事件。ALD和MASLD患者在开发和验证队列中的代表性不足。影响和意义:最近的EASL政策声明强调了基于风险的监测在降低肝细胞癌(HCC)相关死亡人数和监测成本方面的潜力。这项研究弥补了人们对当前HCC风险模型在非病毒性肝病患者中的表现的认识空白,反映了西方国家肝病的流行病学状况。在对这些模型的审查中,我们发现了有关报告标准和偏倚风险的几个关键问题,并证实酒精相关肝病和代谢功能障碍相关脂肪肝患者在模型开发和验证队列中的代表性不足。此外,大多数模型未能考虑到竞争事件的重大风险,从而可能高估了真正的 HCC 风险。本研究强调了这些可能会阻碍风险模型在临床实践中应用的关键问题,并为未来的模型开发研究提出了重要建议。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hepatocellular carcinoma risk scores for non-viral liver disease: A systematic review and meta-analysis
Background & Aims
Hepatocellular carcinoma (HCC) risk prediction models may provide a more personalised approach to surveillance for HCC among patients with cirrhosis. This systematic review aims to summarise the performance of HCC prediction models in patients with non-viral chronic liver disease.
Method
The study was prospectively registered with PROSPERO (ID: CRD42022370078) and reported in accordance with PRISMA guidelines. MEDLINE and Embase databases were searched using a validated search filter for prediction model studies. Two reviewers independently assessed studies for inclusion and risk of bias. Measures of model performance (discrimination and calibration) to assess the risk of HCC at specified time points were identified. A random effects meta-analysis was performed on a subset of studies that reported performance of the same model.
Results
A total of 7,854 studies were identified. After review, 14 studies with a total of 94,014 participants were included; 45% of patients had viral hepatitis, 27% ALD (alcohol-related liver disease) and 19% MASLD (metabolic dysfunction-associated steatotic liver disease). Follow-up ranged from 15.1–138 months. Only one model was developed using a competing risk approach. Age (7 models) and sex (6 models) were the most frequently included predictors. Model discrimination (AUROC or c-statistic) ranged from 0.61–0.947. Only the ‘aMAP’ score (age, male sex, albumin, bilirubin, and platelets) had sufficient external validation for quantitative analysis, with a pooled c-statistic of 0.81 (95% CI 0.80–0.83). Calibration was reported in only 9 of 14 studies. All studies were rated at high risk of bias.
Conclusion
Studies describing risk prediction of HCC in non-viral chronic liver disease are poorly reported, have a high risk of bias and do not account for competing risk events. Patients with ALD and MASLD are underrepresented in development and validation cohorts. These factors remain barriers to the clinical utility and uptake of HCC risk models for those with the most common liver diseases.
Impact and implications:
The recent EASL policy statement emphasises the potential of risk-based surveillance to reduce both hepatocellular carcinoma (HCC)-related deaths and surveillance costs. This study addresses the gap in understanding the performance of current HCC risk models in patients with non-viral liver diseases, reflecting the epidemiological landscape of liver disease in Western countries. In our review of these models we identified several key concerns regarding reporting standards and risk of bias and confirmed that patients with alcohol-related liver disease and metabolic dysfunction-associated steatotic liver disease are underrepresented in model development and validation cohorts. Additionally, most models fail to account for the significant risk of competing events, leading to potential overestimation of true HCC risk. This study highlights these critical issues that may hinder the implementation of risk models in clinical practice and offers key recommendations for future model development studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


