89Zr-immuno-PET 在脑部应用中的新前景 - α-突触核蛋白病
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
摘要
背景最近,利用螯合剂 DFO* 对通过转铁蛋白介导的转运作用在血脑屏障上主动转运的治疗性抗体进行了 89Zr 免疫-PET 成像。在这些研究中,以淀粉样蛋白-β为靶点的阿杜库单抗与转铁蛋白结合单元融合在一起:源自 8D3 的单链 Fab 片段(scFab8D3)。α-突触核蛋白是帕金森病、路易体痴呆症和多系统萎缩症等多种神经退行性疾病的标志性蛋白。α-突触核蛋白的89Zr-免疫PET成像是图像引导药物开发和目标参与评估的重要工具。本研究的目的是通过 89Zr-immuno-PET 在α-突触核蛋白预成纤维(PFF)沉积模型中比较目前使用的两种 8D3 构建物的靶向潜力,即与α-突触核蛋白抗体 HLu-3 融合的单分子 scFab8D3(HLu-3-scFab8D3)和与两个 8D3 单链可变片段(HLu-3-(scFv8D3)2)融合的 HLu-3。HLu-3 和 HIV 靶向药物 B12-scFab8D3 被用作对照组。方法使用 DFO*-NCS 将抗体与 DFO* 连接,然后用 89Zr 进行放射性标记。通过α-突触核蛋白酶联免疫吸附试验和使用表达 mTfR1 的 CHO-S 细胞进行 FACS 分析来评估结合亲和力。首先在细胞外α-突触核蛋白沉积模型中对放射免疫结合剂进行了评估,该模型是通过向 C57Bl/6 WT 小鼠的左侧纹状体颅内注射非调和 PFF 而建立的,同时向对侧部位注射生理盐水作为对照。注射后 1 天、3 天和 7 天进行 PET 成像,然后进行体内外生物分布、自显影和免疫荧光分析。根据这些研究结果,在α-突触核蛋白播种模型中对性能更好的候选抗体进行了类似测试。播种模型具有神经内α-突触核蛋白聚集,是通过向过表达人类野生型α-突触核蛋白的F28tg小鼠的两个纹状体颅内注射超声PFF而建立的。未处理的 F28tg 小鼠和 C57Bl/6 WT 小鼠作为对照。与α-突触核蛋白的结合亲和力没有受损,与 mTfR1 的结合可接受,损失可忽略不计。在沉积模型中使用[89Zr]Zr-HLu-3-scFab8D3和[89Zr]Zr-HLu-3-(scFv8D3)2进行的 PET 成像显示,α-突触核蛋白沉积部位有摄取。不过,不同小鼠的摄取量不同。由于沉积体积较小(约 2 μL),因此无法进行可靠的 PET 定量。免疫荧光显示这两种构建体都与纹状体中的PFF沉积物有特异性的α-突触核蛋白靶参与,这与[89Zr]Zr-B12-scFab8D3对照组形成鲜明对比。出乎意料的是,体外自显影显示一些对照组出现摄取([89Zr]Zr-B12-scFab8D3在对侧纹状体中没有PFF沉积),这可能与注射部位的星形胶质细胞活化有关。与[89Zr]Zr-HLu-3-(scFv8D3)2相比,[89Zr]Zr-HLu-3-scFab8D3的体外和PET脑摄取率更高,因此被选作α-突触核蛋白播种模型的进一步测试。在注射 PFF 的 F28tg 小鼠、F28tg 小鼠和 C57Bl/6 小鼠之间,[89Zr]Zr-HLu-3-scFab8D3 的体内和体外脑摄取量没有明显差异。结论在 PFF 沉积模型中,[89Zr]Zr-HLu-3-scFab8D3 和 [89Zr]Zr-HLu-3-(scFv8D3)2 成功地与α-突触核蛋白靶向结合。PET 成像显示出不同的结果,在某些情况下可以在体内检测到沉积物。由于[89Zr]Zr-HLu-3-scFab8D3在沉积模型中表现较好,因此在α-突触核蛋白内路易体病理学播种模型中进行了进一步研究,结果显示对照组和 PFF 播种小鼠之间没有差异。此外,免疫染色的 F28tg 播种小鼠表现出足够的硬膜内α-突触核蛋白病理学,但没有相应的抗体积累。这些结果突显了通过免疫 PET 对神经内包涵物成像的挑战性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
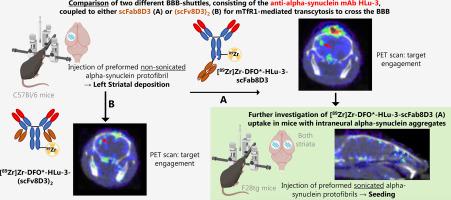
New prospects for 89Zr-immuno-PET in brain applications – Alpha-synucleinopathies
Background
Recently, 89Zr-immuno-PET imaging of therapeutic antibodies, actively transported over the blood-brain-barrier via transferrin-mediated transcytosis, was demonstrated using the chelator DFO*. In these studies, aducanumab targeting amyloid-beta was fused with a transferrin binding unit: a single-chain Fab fragment derived from 8D3 (scFab8D3). Alpha-synuclein is a hallmark protein of several neurodegenerative diseases such as Parkinson's Disease, Lewy-Body-Dementia, and Multiple System Atrophy. 89Zr-immuno-PET imaging of alpha-synuclein can be a valuable tool for image-guided drug development and assessment of target engagement. The goal of this study was to compare two currently used constructs of 8D3 for targeting potential, namely a single moiety of scFab8D3 fused to the alpha-synuclein antibody HLu-3 (HLu-3-scFab8D3) versus HLu-3 fused with two 8D3 single-chain variable fragments (HLu-3-(scFv8D3)2), by 89Zr-immuno-PET in an alpha-synuclein pre-formed fibril (PFF) deposition model. HLu-3 and the HIV-targeting B12-scFab8D3 were used as controls. The best-performing compound was further investigated in an animal model with predominantly intraneural target aggregation.
Methods
Antibodies were conjugated with DFO* using DFO*-NCS and subsequently radiolabeled with 89Zr. Assessment of binding affinity was done by alpha-synuclein ELISA and with FACS analysis using mTfR1 expressing CHO-S cells. Radioimmunoconjugates were first evaluated in an extracellular alpha-synuclein deposition model established by intracranial injection of non-sonicated PFFs into the left striatum of C57Bl/6 WT mice, whereas saline was injected into the contralateral site as control. PET imaging was performed 1, 3, and 7 days post-injection, followed by ex vivo biodistribution, autoradiography and immunofluorescence analysis. Based on the results from these studies, the better-performing antibody candidate was tested similarly in an alpha-synuclein seeding model. The seeding model has intraneural alpha-synuclein aggregation and was established by intracranial injection of sonicated PFFs into both striata of F28tg mice, which overexpress human wild-type alpha-synuclein. Untreated F28tg and C57Bl/6 WT mice served as controls.
Results
The radioimmunoconjugate was produced in sufficient radiochemical yields and purity. There was no impairment of binding affinity towards alpha-synuclein, and acceptable binding with negligible losses to mTfR1. PET imaging with [89Zr]Zr-HLu-3-scFab8D3 and [89Zr]Zr-HLu-3-(scFv8D3)2 in the deposition model showed uptake at the site of alpha-synuclein deposits. However, uptake was variable between mice. Reliable PET quantification was hampered due to the small deposition volume (~2 μL). Immunofluorescence revealed specific alpha-synuclein target engagement of both constructs with PFF deposits in the striatum, in contrast to the [89Zr]Zr-B12-scFab8D3 control. Unexpectedly, ex vivo autoradiography showed uptake in some controls ([89Zr]Zr-B12-scFab8D3 in the contralateral striatum without PFFs), potentially related to astrocyte activation at the injection sites. Ex vivo and PET brain uptake was higher for [89Zr]Zr-HLu-3-scFab8D3 when compared to [89Zr]Zr-HLu-3-(scFv8D3)2 and was therefore selected for further testing in the alpha-synuclein seeding model. No significant difference in in vivo and ex vivo brain uptake of [89Zr]Zr-HLu-3-scFab8D3 between PFF-injected F28tg, F28tg and C57Bl/6 mice was observed. Furthermore, ex vivo immunofluorescence and autoradiography showed no specific alpha-synuclein target engagement.
Conclusions
Successful target engagement of [89Zr]Zr-HLu-3-scFab8D3 and [89Zr]Zr-HLu-3-(scFv8D3)2 with alpha-synuclein was shown in a PFF deposition model. PET imaging showed variable results, and in vivo detection of the depositions was possible in some cases. Due to the better performance in the deposition model, [89Zr]Zr-HLu-3-scFab8D3 was further investigated in an alpha-synuclein seeding model with intraneural Lewy-body pathology, showing no difference between the control groups and PFF-seeded mice. Furthermore, immunostaining of seeded F28tg mice manifested sufficient intraneural alpha-synuclein pathology but no corresponding antibody accumulation. These results underscore the ongoing challenge of imaging intraneural inclusions via immuno-PET.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


