用于直接合成 213Bi 标记生物共轭物的 225Aс/213Bi 发生器
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
摘要
背景213Bi 是一种短寿命放射性核素,目前正试用于各种肿瘤疾病的α治疗。在放射性药物的传统合成过程中,213Bi 的衰变损耗是其广泛应用于医疗领域的一个严重障碍。在这项工作中,我们的目标是开发一种双柱 225Aс/213Bi 发生器,通过中间体 221Fr 的连续循环分离和衰变,将 213Bi 与母体 225Ac 分开积累。 当达到瞬时平衡时,可使用适当的络合剂(包括螯合剂-蛋白质生物共轭物)迅速从发生器中提取 213Bi。方法研究了盐酸和硝酸溶液以及氯化钠、醋酸钠和 DTPA 溶液中 Bi(III)离子在交联葡聚糖凝胶 Sephadex G-25 上的吸附行为。将双功能螯合剂 p-SCN-Bn-DTPA 与表皮生长因子受体特异性抗体尼莫妥珠单抗共轭,并开发了在葡聚糖凝胶介质中合成 207,213Bi-DTPA-Nimotuzumab 的程序。结果得到了吸附在 Sephadex G-25 凝胶上的 Bi(III) 的重量分布比。在 0.15 M NaCl(pH 5.5)溶液中循环四小时后,225Aс/213Bi 发生器的第二个 Sephadex 填充柱中积累了高达 85% 的 213Bi。DTPA-Nimotuzumab 生物轭合物溶液通过第二根柱子后,产生了一部分 213Bi-DTPA-Nimotuzumab 放射免疫轭合物,其放射化学产率为 64 % ± 3 %(n = 6)。结论循环 225Aс/213Bi 发生器提供的最终产品是 213Bi 标记的生物轭合物。传统的合成路线包括发生器挤奶、生物共轭物标记和尺寸排阻纯化需要 20 分钟,而采用本文提出的方法生产 213Bi-DTPA-Nimotuzumab 的时间可缩短至 6-8 分钟。本文章由计算机程序翻译,如有差异,请以英文原文为准。
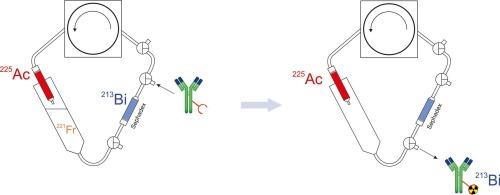
225Aс/213Bi generator for direct synthesis of 213Bi-labeled bioconjugates
Background
213Bi is a short-lived radionuclide currently trialed for alpha therapy of various oncological diseases. A serious obstacle to the wide medical use is decay losses of 213Bi during a conventional synthesis of radiopharmaceuticals. In this work, we aimed to develop a two-column 225Aс/213Bi generator providing the accumulation of 213Bi separately from the parent 225Ac via continuous circular separation and decay of intermediate 221Fr. When attaining the transient equilibrium, 213Bi could be promptly extracted from the generator with an appropriate complexing agent, including chelator-protein bioconjugates.
Methods
Sorption behavior of Bi(III) ions onto the cross-linked dextran gel Sephadex G-25 was studied from solutions of hydrochloric and nitric acid, and from sodium chloride, sodium acetate and DTPA solutions. A bifunctional chelating agent p-SCN-Bn-DTPA was conjugated to an antibody Nimotuzumab specific to the epidermal growth factor receptor, and the procedure of 207,213Bi-DTPA-Nimotuzumab synthesis in the dextran gel medium was developed. The parameters of 225Aс/213Bi generator system were evaluated.
Results
The weight distribution ratios of Bi(III) adsorbed onto the Sephadex G-25 gel were obtained. Up to 85 % of 213Bi was accumulated in the second Sephadex-filled column of 225Aс/213Bi generator after four-hour circulation of 0.15 M NaCl (pH 5.5) solution. Having passed the solution of DTPA-Nimotuzumab bioconjugate through the second column, a fraction of 213Bi-DTPA-Nimotuzumab radioimmunoconjugate was produced with the radiochemical yield of 64 % ± 3 % (n = 6). High radionuclidic and radiochemical purity of product was achieved.
Conclusions
The circulating 225Aс/213Bi generator provides a 213Bi-labeled bioconjugate as a final product. While a conventional synthesis route including generator milking, bioconjugate labeling and size-exclusion purification takes >20 min, the duration of 213Bi-DTPA-Nimotuzumab production by the method proposed in this work is reduced to 6–8 min.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


