一体化制造双金属 PdIn 涂层多孔聚醚砜膜,用于催化甲酸将水中的 NO3 还原成 NH3
IF 5.5
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
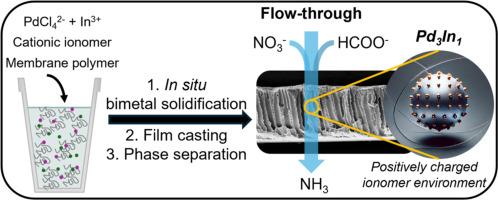
我们采用了一种新颖的一体化方法来制造多孔聚醚砜(PES)膜,其中含有作为催化剂的双金属钯和引入带电表面基团的阳离子离聚物。我们的制备方法是将溶解的钯离子和铟离子原位固化在聚醚砜-离子聚合物浇铸反应溶液中,然后直接在水中通过薄膜浇铸和相分离制备多孔催化膜。我们将制备的膜用于以甲酸(FA)为电子源催化还原水中的 NO3-(50 mg/L,0.81 mM)至 NH3。与 Pd2+ 和 In3+ 或先 Pd 后 In 的顺序固化相比,PdCl42- 和 In3+ 的原位固化产生的 PdIn 物种具有最高的催化活性。此外,Pd3In1-PES 膜中带正电荷的离子膜可将 pH 值为 7 的流体中 NO3- 的转化率从 2.5 mmol/m2h 提高到 17 mmol/m2h,并将 NH3 的选择性从 4 % 提高到 34 %,这可能是通过促进硝酸盐和甲酸根阴离子与催化剂位点之间的相互作用实现的。将流速从 100 L/m2h 降低到 50 L/m2h,NH3 选择性进一步提高到 55%(NH3 生成率为 189 µg/h mg),这说明膜中停留时间的延长促进了 NH3 的形成。此外,我们在流过式工艺中使用 FA 还原 NO3 的电子效率达到了 90%,而在间歇式工艺中仅为 60%,这表明催化剂与 FA 之间的接触时间较短可限制脱氢过程中的过量消耗。最后,我们证明了在 11 小时的流动过程中,NO3- 可持续产生 NH3,并发现有迹象表明 PdIn 可通过电子/氧气转移循环催化 NO3-还原。本文章由计算机程序翻译,如有差异,请以英文原文为准。
All-in-one fabrication of bimetallic PdIn-decorated porous PES membranes for the catalytic flow-through reduction of NO3− to NH3 with formic acid in water
We utilized a novel all-in-one method to fabricate porous polyethersulfone (PES) membranes containing bimetallic PdIn as catalyst and a cationic ionomer to introduce charged surface groups. Our fabrication is based on in situ solidification of dissolved palladium and indium ions in a PES-ionomer casting-reaction solution, directly followed by preparation of porous catalytic membranes through film casting and phase separation in water. We employed as-prepared membranes in the catalytic reduction of NO3− (50 mg/L, 0.81 mM) to NH3 in water using formic acid (FA) as electron source. The in situ solidification of PdCl42− and In3+ generated PdIn species with the highest catalytic activity, compared to Pd2+ and In3+ or a sequential solidification of Pd followed by In. Moreover, positively charged ionomer in a Pd3In1-PES membrane boosted NO3− conversion rate in flow-through at pH 7 from 2.5 to 17 mmol/m2h and NH3 selectivity from 4 % to 34 %, likely by promoting interaction between nitrate and formate anions with catalyst sites. Reducing the flow rate from 100 to 50 L/m2h further enhanced NH3 selectivity to 55 % (NH3 production rate of 189 µg/h mg), illustrating that a longer residence time in the membrane promotes NH3 formation. Additionally, we achieved 90 % electron efficiency for NO3− reduction with FA in flow-through compared to 60 % in batch, highlighting that a short contact time between catalyst and FA limits excess consumption through dehydrogenation. Finally, we demonstrated continuous NH3 production from NO3− for 11 h of flow-through, and found indications that PdIn catalyzes NO3− reduction through an electron/oxygen transfer cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


