Zn:Ti 比率对二氧化碳加氢制甲醇的 PdZn/ZnO-TiO2 催化剂的影响
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究人员使用不同的 Zn 和 Ti 比例(Zn:Ti = 0.5、1、2)合成多孔 ZnO-TiO2 作为 PdZn 合金支撑,以优化二氧化碳加氢制取甲醇的过程。XRD、拉曼和 XPS 分析表明,不同的 Zn 比例会影响晶体结构、氧空位、表面积和 ZnTiO3 相。ZnTiO3 的部分转变发生在 PdZn 周边,与 PdZn 合金形成强烈的界面相互作用。较高的 Zn 含量会促进六方 ZnTiO3 的形成,而较低的比例则有利于立方 ZnTiO3 的形成,从而导致较高的氧空位形成。在低于 250 °C 的温度下,甲醇的选择性很高,最佳生产率为 ∼1326.8 mmolkgcat-1h-1。二氧化碳是 250 ℃ 以上温度下的主要产物,而甲烷和 C - C 偶联反应产生的 C2+ 碳氢化合物则出现在 450 ℃ 时。原位 DRIFTS 分析深入揭示了反应机理,即甲酸*HCOO 和甲酰基*HCO 需要稳定才能加氢生成甲醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
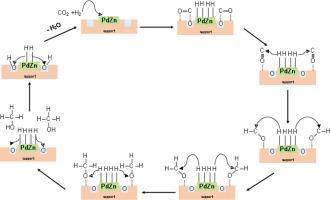
The effect of Zn:Ti ratios on PdZn/ZnO−TiO2 catalysts for CO2 hydrogenation to methanol
Porous ZnO−TiO2 synthesized using different Zn and Ti ratios (Zn:Ti = 0.5, 1, 2) is investigated as PdZn alloy support to optimize methanol production from CO2 hydrogenation. XRD, Raman, and XPS analyses reveal that varying Zn ratios influence crystal structure, oxygen vacancies, surface area and ZnTiO3 phases. The partial transformation into ZnTiO3 occurs on the PdZn perimeter, forming strong interfacial interaction with the PdZn alloy. Higher Zn content promotes hexagonal ZnTiO3, while lower ratios favor cubic ZnTiO3, which leads to higher oxygen vacancy formation. High methanol selectivity was obtained below 250 °C with optimum productivity at ∼1326.8 mmolkgcat-1h-1. CO is the main product at temperatures above 250 °C, while methane and C2+hydrocarbons from the C − C coupling reaction occur at 450 °C. In-situ DRIFTS analysis provides insight into the reaction mechanism that requires stabilization of formate *HCOO and formyl species, *HCO, for hydrogenation to methanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


