中低温 NH3-SCR 用 CTAB 改善 MoCrCeOx 催化剂抗 SO2&H2O 性能的新见解:表面酸和氧物种的调控
IF 5.6
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
氮氧化物的选择性催化还原(NH3-SCR)是最有效的氮氧化物去除技术。提高 SCR 催化剂的抗 SO2/H2O 性能对其工业应用具有重要意义。用柠檬酸法合成的 Mo(0.3)-CrCeOx 催化剂具有优异的耐高浓度 SO2 性能,但耐 H2O 性能较差。本研究采用十六烷基三甲基溴化铵(CTAB)改善了 Mo(0.3)-CrCeOx 催化剂在中低温下的耐 SO2&H2O 性能。研究了 CTAB 改性的机理以及与柠檬酸(CA)法的差异。结果表明,CTAB 催化剂具有较大的比表面积,有利于形成特定的结构强度。CTAB 表面酸性的增强是进一步提高 SCR 活性的原因。调节羟基氧以促进布氏酸位点的活化是抗 SO2&H2O 的关键因素。此外,原位 DRIFTS 结果还揭示了两种催化剂的不同机理。本文提出了溶胶-凝胶催化剂因 SO2&H2O 而失活的原因,并提出了有效的改性策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
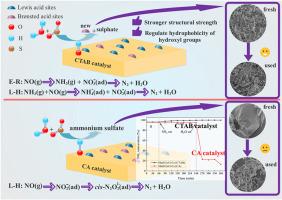
New insights into improved SO2&H2O resistance over MoCrCeOx catalyst by CTAB for medium-low temperatures NH3-SCR: Regulation of surface acid and oxygen species
Selective catalytic reduction (NH3-SCR) of NH3 is the most efficient NOx removal technology. Improving the SO2/H2O resistance of SCR catalyst is of great importance for its industrial application. The Mo(0.3)-CrCeOx catalysts synthesized by the citric acid method exhibited excellent high concentration SO2 resistance but poor H2O tolerance. In this work, cetyltrimethylammonium bromide (CTAB) was used to improve SO2&H2O resistance over Mo(0.3)-CrCeOx catalyst at medium-low temperatures. The mechanism of CTAB modification and the discrepancy to citric acid (CA) method were investigated. The results indicated that the CTAB catalyst had a large specific surface area, and was conducive to forming a specific structural strength. The enhancement of surface acidity of CTAB is the reason for the further enhancement of SCR activity. The regulation of hydroxyl oxygen to promote the activation of Brønsted acid sites is the key factor to SO2&H2O resistance. In addition, the in-situ DRIFTS results revealed the different mechanisms of the two catalysts. This paper raised the deactivation reason of sol-gel catalyst caused by SO2&H2O and presented an effective modification strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


