通过绿色合成 PET 衍生的金属有机框架实现高效光催化染料降解的变废为宝战略
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
被染料污染的废水会对环境和健康造成严重危害,包括耗氧和致癌。光催化技术利用丰富的太阳能驱动化学反应,提供了一种可持续的高效解决方案,是一种经济有效的水处理方法。金属有机框架(MOFs)以其高比表面积、多孔性和化学可调性而著称,是此类应用中特别有前景的材料。在本研究中,我们开发了一种将聚对苯二甲酸乙二醇酯(PET)瓶转化为对苯二甲酸的绿色合成方法,然后利用对苯二甲酸合成 PET-MIL-101(Cr)(简称 PM-101(Cr))。合成过程避免使用 HF 或 DMF 等有机溶剂,以防止二次污染。通过硝化进一步改性 PM-101(Cr),生成 PET-NO2-MIL-101(Cr) (PO-101(Cr)),随后将其还原成胺功能化 PET-NH2-MIL-101(Cr) (PH-101(Cr))。PH-101(Cr) 在蓝光照射 4 小时内的染料去除率超过 95%,超过 PM-101(Cr)。此外,经过四次催化循环后,PH-101(Cr) 的还原效率在 6 小时内保持在 95% 以上,这表明它具有有效处理染料的强大潜力。总之,PH-101(Cr) 可在蓝色 LED 光下高效降解亚甲基蓝染料,实现废物回收和环境修复,提高可持续性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
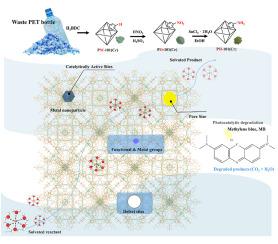
Waste-to-resource strategy through green synthesis of PET-derived metal-organic frameworks for efficient photocatalytic dye degradation
Wastewater contaminated with dyes poses significant environmental and health hazards, including oxygen depletion and carcinogenesis. Photocatalytic technology offers a sustainable and efficient solution by harnessing abundant solar energy to drive chemical reactions, making it a cost-effective method for water treatment. Metal-Organic Frameworks (MOFs), known for their high surface area, porosity, and chemical tunability, are particularly promising materials for such applications. In this study, we developed a green synthesis method to repurpose polyethylene terephthalate (PET) bottles into terephthalic acid, which was then used to synthesize PET-MIL-101(Cr) (denoted as PM-101(Cr)). The synthesis process avoids the use of organic solvents such as HF or DMF to prevent secondary pollution. Further modification of PM-101(Cr) through nitration produced PET-NO2-MIL-101(Cr) (PO-101(Cr)), which was subsequently reduced to form amine-functionalized PET-NH2-MIL-101(Cr) (PH-101(Cr)). PH-101(Cr) demonstrated a dye removal efficiency of over 95 % within 4 h of blue light irradiation, surpassing the performance of PM-101(Cr). Additionally, after four catalytic cycles, PH-101(Cr) maintained a reduction efficiency above 95 % for 6 h, indicating its robust potential for effective dye treatment. In summary, PH-101(Cr) efficiently degrades methylene blue dye under blue LED light, achieving both waste recycling and environmental remediation for improved sustainability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


