机理依赖性山梨醇积累支持生物分子凝聚物
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
凝结的蛋白质液滴能调节许多细胞功能,但调节其形成的生理条件在很大程度上仍未得到研究。增加我们对这些机制的了解至关重要,因为无法控制凝聚物的形成和动态会导致许多疾病。在这里,我们提供了基质硬化促进体内生物分子凝聚的证据。我们证明,细胞外基质将机械线索与控制山梨醇的葡萄糖代谢联系起来。反过来,山梨醇又作为一种天然的挤压剂促进生物分子的凝结。我们利用硅学模拟和体外试验证实,生理范围内山梨醇浓度的变化,而非葡萄糖浓度的变化,足以调节生物分子凝聚。因此,对细胞内山梨醇浓度的药理学和遗传学操作可以调节乳腺癌--一种机械依赖性疾病--中的生物分子凝聚物。我们认为,山梨醇是一种对机械敏感的代谢物,它能使蛋白质凝结,从而控制受机械调控的细胞功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
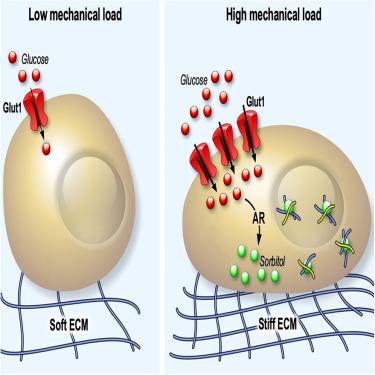
Mechano-dependent sorbitol accumulation supports biomolecular condensate
Condensed droplets of protein regulate many cellular functions, yet the physiological conditions regulating their formation remain largely unexplored. Increasing our understanding of these mechanisms is paramount, as failure to control condensate formation and dynamics can lead to many diseases. Here, we provide evidence that matrix stiffening promotes biomolecular condensation in vivo. We demonstrate that the extracellular matrix links mechanical cues with the control of glucose metabolism to sorbitol. In turn, sorbitol acts as a natural crowding agent to promote biomolecular condensation. Using in silico simulations and in vitro assays, we establish that variations in the physiological range of sorbitol concentrations, but not glucose concentrations, are sufficient to regulate biomolecular condensates. Accordingly, pharmacological and genetic manipulation of intracellular sorbitol concentration modulates biomolecular condensates in breast cancer—a mechano-dependent disease. We propose that sorbitol is a mechanosensitive metabolite enabling protein condensation to control mechano-regulated cellular functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


