SARS-CoV-2 在宿主内持续存在的后果
IF 103.3
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
SARS-CoV-2 会引起急性呼吸道感染,大多数人在不到一个月的时间内就会痊愈。然而,一些免疫力严重低下的人却无法清除病毒,导致呼吸道持续感染,病毒滴度很高。在一部分病例中,SARS-CoV-2 的持续复制会导致适应性突变的加速积累,从而逃避中和抗体的作用并增强细胞感染。这可能导致广泛变异的 SARS-CoV-2 变异体的进化,并在变异体进化的时间上引入偶然因素,因为变异体的形成可能取决于单个人的进化。长期 COVID 是否也是由持续复制的 SARS-CoV-2 引起的,这一点还存在争议。一个证据是,在 SARS-CoV-2 感染从上呼吸道清除后很长时间,在不同的身体分区中检测到 SARS-CoV-2 RNA 和蛋白质。然而,迄今为止,尚未从免疫功能正常的长期 COVID 感染者体内培养出具有复制能力的病毒。在本综述中,我们将探讨病毒持续存在的机制、持续感染中宿主内的进化、持续感染与 SARS-CoV-2 变异的联系以及 SARS-CoV-2 持续存在在长 COVID 中可能扮演的角色。因此,了解持续感染可能会解决 COVID-19 病理生理学中仍不清楚的许多问题,并可能对其他新出现的病毒产生影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
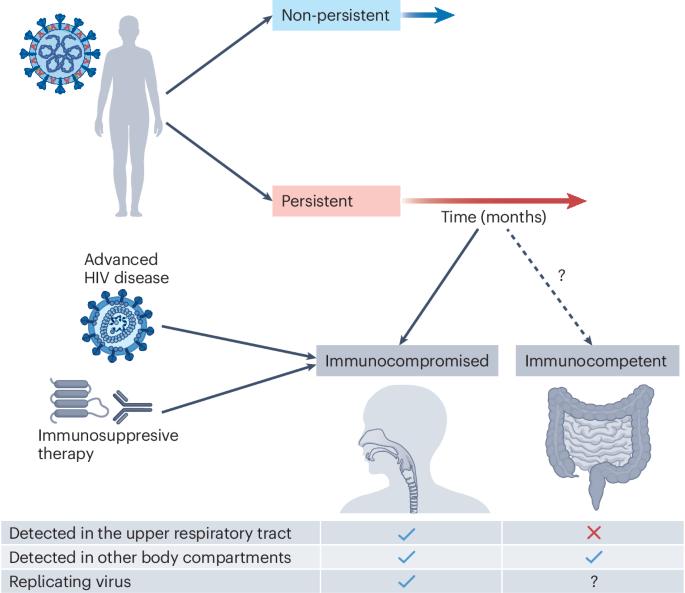
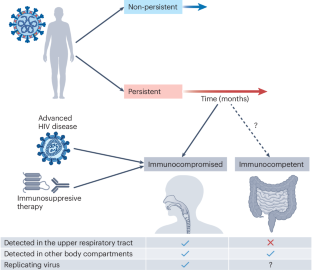
The consequences of SARS-CoV-2 within-host persistence
SARS-CoV-2 causes an acute respiratory tract infection that resolves in most people in less than a month. Yet some people with severely weakened immune systems fail to clear the virus, leading to persistent infections with high viral titres in the respiratory tract. In a subset of cases, persistent SARS-CoV-2 replication results in an accelerated accumulation of adaptive mutations that confer escape from neutralizing antibodies and enhance cellular infection. This may lead to the evolution of extensively mutated SARS-CoV-2 variants and introduce an element of chance into the timing of variant evolution, as variant formation may depend on evolution in a single person. Whether long COVID is also caused by persistence of replicating SARS-CoV-2 is controversial. One line of evidence is detection of SARS-CoV-2 RNA and proteins in different body compartments long after SARS-CoV-2 infection has cleared from the upper respiratory tract. However, thus far, no replication competent virus has been cultured from individuals with long COVID who are immunocompetent. In this Review, we consider mechanisms of viral persistence, intra-host evolution in persistent infections, the connection of persistent infections with SARS-CoV-2 variants and the possible role of SARS-CoV-2 persistence in long COVID. Understanding persistent infections may therefore resolve much of what is still unclear in COVID-19 pathophysiology, with possible implications for other emerging viruses. In this Review, Sigal et al. explore SARS-CoV-2 persistence mechanisms, the frequency of persistent infections, their role in accelerated evolution and their link to variant emergence hot spots. The Review also addresses symptoms, management and the connection between persistence and post-COVID conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


