揭示二元半导体纳米团簇的形成机制:双壳结构硫化铜纳米团簇的两步形成途径
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
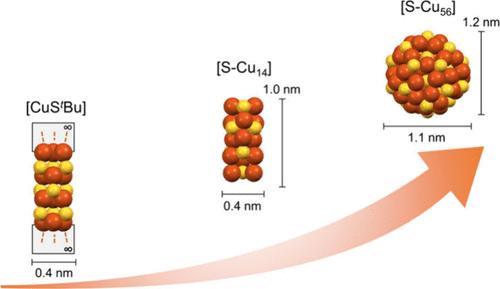
这项研究是揭示原子精确二元半导体纳米团簇形成机制的重要一步。在这项研究中,我们开发了一种酸辅助 C-S 键裂解方法,即在 Cu2O 作为催化剂存在的情况下,在适当酸的辅助下,硫代金属前体中的 C-S 键可以很容易地被裂解以释放出 S2-。这一过程自发地促进了[-Cu-S-Cu-]框架的形成,并推动结构成长为高核度组装体。具体来说,通过使用硫代叔丁基铜(I)([CuStBu]∞)和羧酸 CH2═CHCOOH 作为铜/硫前驱体和 C-S 键 "剪刀",高产率地有效制备出了具有双壳构型的高核度纳米簇合物 [S-Cu56](Cu56S12(OOCCH═CH2)12(SC(CH3)3)20)。重要的是,[CuStBu]∞ 前体和中间体[S-Cu14](Cu14(StBu)8(OOCCH═CH2)6)团簇也被成功分离出来并从结构上进行了表征,从而最终建立了[S-Cu56]纳米团簇的两步形成途径。此外,与含有 Cu(0) 或 Cu(I) 的金属纳米簇的传统还原合成路线不同,酸辅助 C-S 键裂解方法代表了组成金属的氧化过程,在硫化铜纳米簇中产生了高电荷的 Cu(II) 阳离子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling the Formation Mechanism for Binary Semiconductor Nanoclusters: a Two-Step Pathway to a Double-Shell Structured Copper Sulfide Nanocluster
This work represents an important step in the quest to unveil the formation mechanism of atomically precise binary semiconductor nanoclusters. In this study, we develop an acid-assisted C–S bond cleavage approach, wherein the C–S bonds in the metal thiolate precursor can be readily cleaved to release S2– with the assistance of a suitable acid in the presence of Cu2O as the catalyst. This process spontaneously fosters the formation of a [−Cu–S–Cu−] framework and promotes the structural growth into a high nuclearity assembly. Specifically, by employing Cu(I) tert-butyl thiolate ([CuStBu]∞) and carboxylate acid CH2═CHCOOH as the copper/sulfur precursor and C–S bond “scissor”, a high-nuclearity nanocluster [S–Cu56] (Cu56S12(OOCCH═CH2)12(SC(CH3)3)20) featuring a double-shell configuration has been effectively prepared in high yield. Importantly, the [CuStBu]∞ precursor and the intermediate [S–Cu14] (Cu14(StBu)8(OOCCH═CH2)6) cluster have also been successfully isolated and structurally characterized, which ultimately enables the establishment of a two-step formation pathway for the [S–Cu56] nanocluster. Furthermore, in contrast to conventional reduction synthetic routes for metal nanoclusters containing Cu(0) or Cu(I), the acid-assisted C–S bond cleavage approach represents an oxidation process with respect to the constituent metals, yielding highly charged Cu(II) cations in the copper sulfide nanocluster.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


