哥斯达黎加五种小鼠毒液的自上而下蛋白质组学:富含磷脂酶 A2- 与富含三指毒素表型的比较组成。
IF 2.6
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
珊瑚蛇属包括分布在美洲大陆的 80 多个物种。它们产生的毒液具有强烈的神经毒性,作用于神经肌肉接头,可能导致呼吸麻痹和死亡。珊瑚蛇毒中的绝大多数蛋白质属于三指毒素(3FTx)和第一组磷脂酶 A2(PLA2)家族。以前使用 "自下而上 "的蛋白质组学策略进行的研究揭示了毒素表达的组成二分法,不同的珊瑚蛇物种在其毒液中显示出 3FTx 或 PLA2 蛋白的优势,这可能与该属辐射的系统地理结构有关。自上而下 "蛋白质组学(TDP)允许在高分辨率质谱仪中直接分析完整的蛋白质,避免了自下而上方法固有的 "肽到蛋白质推断问题 "的局限性。在这里,我们使用 TDP 方法分析了哥斯达黎加六种小龙鼠中五种的毒液。结果揭示了这些物种之间共有的毒液蛋白形式,并进一步揭示了这些毒液成分的复杂性及其与 3FTx/PLA2 二分法之间的关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
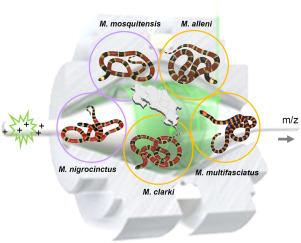
Top-down proteomics of venoms from five Micrurus species from Costa Rica: comparative composition of phospholipase A2-rich vs three-finger toxin-rich phenotypes
Coralsnakes of the genus Micrurus include more than 80 species distributed in the American continent. They produce potent neurotoxic venoms acting at the neuromuscular junction and potentially leading to respiratory paralysis and death. The vast majority of proteins in coralsnake venoms belong to the three-finger toxin (3FTx) and the group I phospholipase A2 (PLA2) families. Previous studies using ‘bottom-up’ proteomic strategies have revealed a compositional dichotomy of toxin expression by which different Micrurus species display a predominance of either 3FTx or PLA2 proteins in their venoms, possibly linked to the phylogeographic structure of the genus radiation. ‘Top-down’ proteomics (TDP) allows the direct analysis of intact proteins in a high resolution mass spectrometer, circumventing the limitations of the ‘peptide-to-protein inference problem’ inherent to the bottom-up approach. Here, we analyzed the venoms of five out of the six Micrurus species that inhabit Costa Rica, by using a TDP approach. Results unveil venom proteoforms that are shared between these species, and provide additional insights into the variable compositional complexity of these venoms and relationships to their 3FTx/PLA2 dichotomy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


