关于 225Ac 标记的二氧化钛纳米粒子释放 221Fr 和 213Bi 子代的体外和体内研究。
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
摘要
背景:靶向α疗法(TAT)是一种有效的癌症治疗方法。为了最大限度地提高疗效并减少副作用,载体必须将放射性核素输送到靶组织。α疗法中使用的大多数核素都会通过α级联衰变,产生几个具有足够能量的放射性子核,从而逃离原始载体。因此,研究这些子原子对于寻找新的载体至关重要。纳米粒子因其结构而具有作为载体的潜力,可以防止子原子逸出,减少对非目标组织的辐射照射。这项工作的重点是确定 225Ac 标记的二氧化钛纳米粒子衰变所释放的 221Fr 和 213Bi 的活性:结果:对二氧化钛纳米粒子的标记表明,225Ac及其衍生物221Fr和213Bi的吸附率很高,对所有测得的放射性核素而言,92%以上的放射性活度都吸附在纳米粒子表面。然而,在准动态体外系统中,221Fr 和 213Bi 释放的放射性活度与纳米粒子的浓度密切相关,在生理盐水溶液中,浓度为 1 毫克/毫升的纳米粒子释放的放射性活度为 15%,浓度为 10 微克/毫升的纳米粒子释放的放射性活度约为 50%。213Bi 的释放活性较低,在浓度为 0.05、0.025 和 0.01 毫克/毫升时,最大值约为 20%。在各种生物基质中测试了 225Ac 及其后代的泄漏情况。48 小时后,在生理盐水中测得的最小释放活性约为 10%,而在血清和血浆中测得的最大释放活性为 20%。释放到介质中的 225Ac 数量极少(213Bi 与 225Ac 达到平衡):该研究验证了标记的二氧化钛纳米粒子释放 225Ac 原物的可能性。实验确定了释放活性与纳米粒子浓度和生物环境的关系。结果表明,制备的 225Ac@TiO2 NPs 具有很高的稳定性,并有可能随着时间的推移释放出原生物质。体内研究证实了我们的假设。所获得的数据表明,子原子可以从原始载体中逸出,并在生物体内遵循其自身的生物路径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
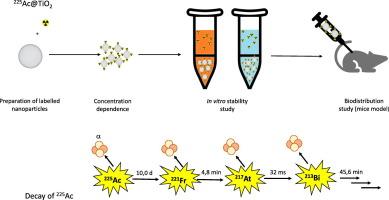
In vitro and in vivo study of 221Fr and 213Bi progeny release from the 225Ac-labelled TiO2 nanoparticles
Background
Targeted alpha therapy (TAT) is an effective option for cancer treatment. To maximize its efficacy and minimize side effects, carriers must deliver radionuclides to target tissues. Most of the nuclides used in TAT decay via the alpha cascade, producing several radioactive daughter nuclei with sufficient energy to escape from the original carrier. Therefore, studying these daughter atoms is crucial in the search for new carriers. Nanoparticles have potential as carriers due to their structure, which can prevent the escape of daughter atoms and reduce radiation exposure to non-target tissues. This work focuses on determining the released activity of 221Fr and 213Bi resulting from the decay of 225Ac labelled TiO2 nanoparticles.
Results
Labelling of TiO2 nanoparticles has shown high sorption rates of 225Ac and its progeny, 221Fr and 213Bi, with over 92 % of activities sorbed on the nanoparticle surface for all measured radionuclides. However, in the quasi-dynamic in vitro system, the released activity of 221Fr and 213Bi is strongly dependent on the nanoparticles concentration, ranging from 15 % for a concentration of 1 mg/mL to approximately 50 % for a nanoparticle concentration of 10 μg/mL in saline solution. The released activities of 213Bi were lower, with a maximum value of around 20 % for concentrations of 0.05, 0.025, and 0.01 mg/mL. The leakage of 225Ac and its progeny was tested in various biological matrices. Minimal released activity was measured in saline at around 10 % after 48 h, while the maximum activity was measured in blood serum and plasma at 20 %. The amount of 225Ac released into the media was minimal (<3 %). The in vitro results were confirmed in a healthy mouse model. The difference in %ID/g was clearly visible immediately after dissection and again after 6 h when 213Bi reached equilibrium with 225Ac.
Conclusion
The study verified the potential release of 225Ac progeny from the labelled TiO2 nanoparticles. Experiments were performed to determine the dependence of released activity on nanoparticle concentration and the biological environment. The results demonstrated the high stability of the prepared 225Ac@TiO2 NPs and the potential release of progeny over time. In vivo studies confirmed our hypothesis. The data obtained suggest that the daughter atoms can escape from the original carrier and follow their own biological pathways in the organism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


