艾兰通以KMT2A-MEN1复合物为靶点抑制骨肉瘤的肺转移
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:肺转移是骨肉瘤(OS)患者的主要死因,迫切需要新的药物。表观遗传重编程是最近提出的恶性肿瘤的标志;因此,靶向表观遗传酶可能为骨肉瘤肺转移提供一种新的治疗策略。我们最近报道了一种从中药植物Ailanthus altissima中分离出来的天然产物--Ailanthone(AIL)能抑制OS细胞的生长并诱导实质性的代谢变化;然而,其直接靶点仍不清楚:研究设计:在Saos-2和U-2OS OS细胞中鉴定AIL的直接靶蛋白和下游信号通路。研究设计:在Saos-2和U-2OS OS细胞中鉴定了AIL的直接靶蛋白和下游信号通路,并利用小鼠模型研究了AIL对OS肺转移的体内影响:方法:采用新型表面等离子体共振-高效液相色谱-质谱(SPR-HPLC-MS)分析法确定AIL在OS中的直接靶标。通过细胞热转移实验、分子对接分析、酶活性实验、qRT-PCR、Western印迹、染色质免疫沉淀实验和反向实验来确认AIL的靶点和下游通路。结果表明:组蛋白-N-赖氨酸-N-甲基化抑制剂的作用靶点和下游通路得到了确认,并通过肿瘤异种移植模型验证了其在体内的疗效和机制:结果:组蛋白-赖氨酸N-甲基转移酶2A(KMT2A)及其支架蛋白menin(MEN1)被确定为AIL在OS中的直接靶蛋白。AIL诱导了KMT2A-MEN1复合物的自噬降解。此外,AIL还抑制了细胞内H3K4甲基转移酶的活性,并从表观遗传学上抑制了丝氨酸生物合成途径(SSP)基因的转录。此外,在小鼠模型中,AIL抑制了OS肺转移,并下调了KMT2A、MEN1和SSP:结论:这项研究表明,AIL靶向KMT2A-MEN1复合物并抑制SSP,从而抑制OS肺转移。值得注意的是,AIL 展现了不同于现有抗 OS 药物的新作用机制。基于这些发现,我们提出了一种通过靶向表观遗传酶和癌症代谢来治疗OS的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
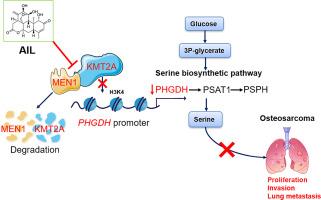
Ailanthone targets the KMT2A-MEN1 complex to suppress lung metastasis of osteosarcoma
Background
Lung metastasis is the leading cause of death in patients with osteosarcoma (OS), and new drugs are urgently needed. Epigenetic reprogramming is a recently proposed hallmark of malignancy; therefore, targeting epigenetic enzymes might provide a novel therapeutic strategy for OS lung metastasis. We recently reported that ailanthone (AIL), a natural product isolated from the Chinese medicinal plant Ailanthus altissima, inhibits OS cell growth and induces substantial metabolic changes; however, its direct targets remain unclear.
Purpose
To identify the direct targets of AIL in OS and to explore the effects of AIL on OS lung metastasis in vivo.
Study design
Direct target proteins of AIL and downstream signaling pathways were identified in Saos-2 and U-2OS OS cells. The in vivo effects of AIL on OS lung metastasis were investigated using a mouse model.
Methods
A novel surface plasmon resonance-high-performance liquid chromatography-mass spectrometry (SPR-HPLC-MS) assay was used to determine direct targets of AIL in OS. A cellular thermal shift assay, molecular docking analysis, enzyme activity assay, qRT-PCR, western blotting, chromatin immunoprecipitation assay, and reverse tests were performed to confirm the target and downstream pathway of AIL. A tumor xenograft model was used to verify the efficacy and mechanisms in vivo.
Results
Histone-lysine N-methyltransferase 2A (KMT2A) together with its scaffold protein menin (MEN1) were identified as direct target proteins of AIL in OS. AIL induced the autophagic degradation of the KMT2A-MEN1 complex. Moreover, AIL inhibited intracellular H3K4 methyltransferase activity and epigenetically inhibited the transcription of genes in the serine biosynthetic pathway (SSP). Furthermore, AIL suppressed OS lung metastasis and downregulated KMT2A, MEN1, and SSP in mouse models.
Conclusion
This work showed that AIL targets the KMT2A-MEN1 complex and inhibits SSP to suppress OS lung metastasis. Notably, AIL exhibits new mechanisms of action, distinct from those of existing anti-OS drugs. On the basis of these findings, we proposed a novel strategy to treat OS by targeting epigenetic enzymes and cancer metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


