康莱特通过铁氧化作用缓解肺鳞癌。
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
康莱特是一种主要由多不饱和脂肪酸(PUFAs)组成的化合物,在中国用于临床治疗腺癌非小细胞肺癌(NSCLC)已有几十年的历史。然而,其在治疗鳞状非小细胞肺癌中的疗效和特异性机制仍有待探索。在这项研究中,我们证明了与铁离子联合处理可通过诱导NCL-H1703(一种人肺鳞癌细胞系)的铁变态反应,显著增强康莱特的细胞毒性作用。机理研究发现,kanglaite 会诱导线粒体功能障碍,导致活性氧(ROS)过量产生,这对于诱导铁中毒至关重要。进一步的分析表明,kanglaite 会抑制 PI3K/AKT 信号通路,导致 IP3 生成增加。IP3 随后与内质网(ER)钙通道 IP3R 结合并激活 IP3R,加剧了从 ER 到线粒体的过度钙转移。线粒体钙过载会导致线粒体功能障碍,并增加 ROS 的产生。为了优化铁离子和康莱特的协同作用,我们开发了一种介孔二氧化硅纳米药物递送系统,该系统共同负载了康莱特和 Fe3O4,具有几个显著的优点,包括减少药物剂量和加快治疗起效。最后,在 NCL-H1703 异种移植模型中,DMSN/Fe3O4-Kanglaite 纳米药物显著抑制了肿瘤的生长。总之,我们确定了康莱特在治疗鳞状非小细胞肺癌中的功能和机制,并开发了一种 DMSN/Fe3O4-Kanglaite 纳米药物,为治疗鳞状非小细胞肺癌提供了一种卓越的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
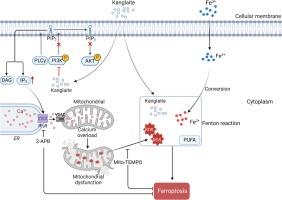
Kanglaite alleviates lung squamous cell carcinoma through ferroptosis
Kanglaite, a compound predominantly composed of polyunsaturated fatty acids (PUFAs), has been employed in the clinical treatment of adenocarcinoma non-small cell lung cancer (NSCLC) in China for decades. However, its therapeutic efficacy and specific mechanism in the treatment of squamous NSCLC remains unexplored. In this study, we demonstrate that the co-treatment with ferric ion significantly enhances the cytotoxic effects of kanglaite by inducing ferroptosis in NCL-H1703, a cell line of human lung squamous cell carcinoma. Mechanistic investigations reveal that kanglaite induces mitochondrial dysfunction resulting in reactive oxygen species (ROS) excessive production, which is critical for the induction of ferroptosis. Further analysis shows that kanglaite suppresses the PI3K/AKT signaling pathway, leading to increased IP3 generation. IP3 subsequently binds to and activates IP3R, an endoplasmic reticulum (ER) calcium channel, exacerbating the excessive calcium transfer from the ER to mitochondria. The overloaded mitochondrial calcium contributes to its dysfunction and elevates ROS production. To optimize the synergistic effects of ferric ion and kanglaite, we develop a mesoporous silica-based nanodrug delivery system co-loaded with Kanglaite and Fe3O4, which offers several notable advantages, including reduced drug dosage and a faster therapeutic onset. Finally, in an NCL-H1703 xenograft model, the DMSN/Fe3O4-Kanglaite nanodrug significantly inhibited tumor growth. In conclusion, we identified the function and mechanism of kanglaite in treatment of squamous NSCLC and have developed a DMSN/Fe3O4-Kanglaite nanodrug, providing a superior therapeutic approach for the treatment of squamous NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


