开发和评估用于结肠特异性输送亲水性生物材料的创新型肠溶胶囊。
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
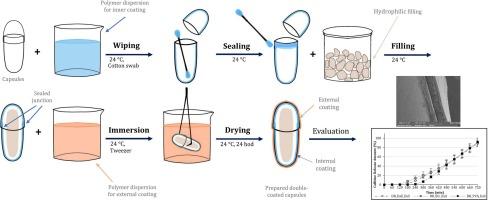
研究目的本研究旨在设计和评估一种肠溶硬质胶囊剂型,用于向胃肠道远端靶向输送生物材料,如粪便微生物群移植(FMT)或活微生物。方法:使用基于 HPMC 和结冷胶的硬明胶胶囊和 DRcapsTM 胶囊来封装含有咖啡因或不溶性氧化铁混合物的亲水性体温液化明胶水凝胶。测试了内涂层(乙基纤维素、Eudragit® E 和聚醋酸乙烯酯)和外涂层(Eudragit® S、Acryl-EZE® 和细胞色素)的不同聚合物组合。外包衣可防止胃酸环境,而内包衣则可防止液体亲水填充物通过。使用标准崩解法和改进的缓释剂型溶出法对包衣胶囊进行了评估:结果:将 DRcapsTM 胶囊的合适内涂层(乙基纤维素 1.0%)和外涂层(Eudragit® S 20.0%)与擦拭和浸泡法相结合,可实现结肠释放时间。虽然大多数包衣胶囊都能满足延迟释放的制药要求,但有一种包衣组合脱颖而出。结肠释放时间由溶解法测定的可溶性咖啡因的溶解(120-720 分钟)和崩解法测定的不溶性氧化铁的释放(480 分钟后)来表示。这一令人鼓舞的结果表明了该成分的适用性和保护内容物直至其释放的潜力,为结肠靶向给药系统的未来及其在制药和生物医学领域的潜力带来了希望:创新而简便的胶囊包衣为将药物(尤其是 FMT 水悬液)靶向送入消化道(最好是结肠)提供了巨大的潜力。这种给药方法非常可靠,不会受到内部或外部包衣量的很大影响。它可以在普通实验室进行,无需专门的个人和个性化治疗设备,是一种切实可行的给药方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development and evaluation of innovative enteric-coated capsules for colon-specific delivery of hydrophilic biomaterials
Objective
This research aims to design and evaluate an enteric-coated hard capsule dosage form for targeted delivery of biological materials, such as FMT (fecal microbiota transplant) or live microbes, to the distal parts of the GIT. The capsules are designed to be internally protected against destruction by hydrophilic filling during passage through the digestive tract.
Methods
Hard gelatin capsules and DRcapsTM capsules based on HPMC and gellan were used to encapsulate a hydrophilic body temperature-liquefying gelatin hydrogel with caffeine or insoluble iron oxide mixture. Different combinations of polymers were tested for the internal (ethylcellulose, Eudragit® E, and polyvinyl acetate) and external (Eudragit® S, Acryl-EZE®, and cellacefate) coating. The external protects against the acidic gastric environment, while the internal protects against the liquid hydrophilic filling during passage. Coated capsules were evaluated using standard disintegration and modified dissolution methods for delayed-release dosage forms.
Results
Combining suitable internal (ethylcellulose 1.0 %) and external (Eudragit® S 20.0 %) coating of DRcapsTM capsules with the wiping and immersion method achieved colonic release times. While most coated capsules met the pharmaceutical requirements for delayed release, one combination stood out. Colonic times were indicated by the dissolution of soluble caffeine (during 120–720 min) measured by the dissolution method, and capsule rupture was indicated by the release of insoluble iron oxide (after 480 min) measured by the disintegration method. This promising result demonstrates the composition’s suitability and potential to protect the content until it’s released, inspiring hope for the future of colon-targeted delivery systems and its potential for the pharmaceutical and biomedical fields.
Conclusion
Innovative and easy capsule coatings offer significant potential for targeted drugs, especially FMT water suspension, to the GIT, preferably the colon. The administration method is robust and not considerably affected by the quantity of internal or external coatings. It can be performed in regular laboratories without specialized individual and personalized treatment equipment, making it a practical and feasible method for drug delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


