免疫特征与炎症蛋白的结合揭示了自身免疫性肝病的发病机制:孟德尔随机化研究
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
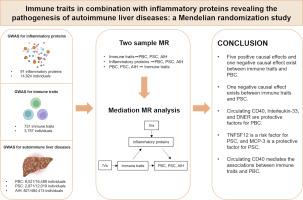
背景:先前的观察性研究表明,免疫细胞、炎症蛋白与自身免疫性肝病(AILD)之间存在关系,但它们之间的因果关系仍存在争议。因此,我们旨在阐明它们之间的因果关系:我们进行了一项全面的孟德尔随机化(MR)分析,以明确731个免疫特征、91个循环炎症蛋白与AILD(包括原发性胆汁性胆管炎(PBC)、原发性硬化性胆管炎(PSC)和自身免疫性肝炎(AIH))之间的因果关系。我们采用了两步磁共振分析来探讨循环炎症蛋白的介导作用。此外,我们还进行了敏感性分析,以评估结果的稳健性:结果:IgD+CD24+B细胞上的CD27、IgD-CD38dimB细胞上的CD27、未切换记忆B细胞上的CD27、切换记忆B细胞上的CD27和CD24+CD27+B细胞上的CD27是PBC的危险因素。然而,我们发现 IgD-CD27-B 细胞上的 CD25 对 PBC 有保护作用,静息 CD4+Treg 细胞上的 CD28 对 PSC 有保护作用。循环中的CD40、白细胞介素-33、Delta和Notch样表皮生长因子相关受体是PBC的保护因子。此外,CD40介导了免疫特征与PBC之间的关联,介导的比例从18.3%到35.4%不等。肿瘤坏死因子超家族成员 12 被确定为 PSC 的风险因素,而单核细胞趋化蛋白 3 被确定为 PSC 的保护因素。此外,PBC 和 PSC 对 11 种免疫特征也有影响,这被认为是它们的后果。我们发现免疫特征、循环炎症蛋白和 AIH 之间没有因果关系。敏感性分析表明我们的结果是可靠的:我们的研究结果证明了免疫特质和炎症蛋白在 PBC 和 PSC 中的因果作用,揭示了它们的发病机制。有必要研究免疫细胞和炎症蛋白影响 AILD 发生的具体机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Immune traits in combination with inflammatory proteins revealing the pathogenesis of autoimmune liver diseases: A Mendelian randomization study
Background
Prior observational research has shown relationships between immune cells, inflammatory proteins, and autoimmune liver diseases (AILD), but their causal associations remain controversial. Therefore, we aimed to clarify the causal association between them.
Methods
We carried out a comprehensive Mendelian randomization (MR) analysis to clarify causal associations between 731 immune traits, 91 circulating inflammatory proteins, and AILD, including primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH). A two-step MR analysis was used to explore the mediating role of circulating inflammatory proteins. Additionally, we performed sensitivity analyses to evaluate the robustness of the results.
Results
CD27 on IgD+ CD24+ B cell, CD27 on IgD− CD38dim B cell, CD27 on unswitched memory B cell, CD27 on switched memory B cell, and CD27 on CD24+ CD27+ B cell were risk factors for PBC. However, we detected protective effects of CD25 on IgD− CD27− B cell against PBC and CD28 on resting CD4+ Treg cell against PSC. Circulating CD40, Interleukin-33, and Delta and Notch-like epidermal growth factor-related receptor were protective factors for PBC. Furthermore, CD40 mediated the association between immune traits and PBC, with the mediated proportions ranging from 18.3 % to 35.4 %. Tumor necrosis factor superfamily member 12 was identified as a risk factor for PSC, and monocyte chemotactic protein 3 was identified as a protective factor for PSC. Additionally, PBC and PSC had effects on eleven immune traits, which are suggested to be the consequences of them. We found no causal association between immune traits, circulating inflammatory proteins, and AIH. Sensitivity analyses demonstrated our results were robust.
Conclusions
Our results demonstrate the causal roles of immune traits and inflammatory proteins in PBC and PSC, which reveals their pathogenesis. It is necessary to investigate the specific mechanism by which immune cells and inflammatory proteins affecting the occurrence of AILD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


