线粒体靶向青蒿琥酯-血红素共轭物:连接体调节的细胞渗透性、血红素亲和力和抗癌活性
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
血红素在癌细胞的线粒体中含量丰富,是青蒿素(ART)抗癌活性的关键靶点。目前增强青蒿素抗癌活性的策略仅关注将青蒿素输送到血红素丰富的亚细胞定位,而忽视了青蒿素与血红素相互作用的决定性影响。在这里,我们提出了一种巧妙的策略,它能协同线粒体靶向给药和连接体介导的药物构象调控,从而显著增强 ART 的抗癌活性。通过使用不同的连接体将青蒿素(ART)与线粒体靶向药物瑞香素(Rhein,R)策略性地共轭,我们旨在精确调节共轭物的构象。综合计算分析表明,具有最佳连接体长度(C4)的共轭物具有良好的构象,有利于细胞渗透,并与血红素和铁离子具有最高的结合亲和力。此外,它在体外和体内都表现出卓越的肿瘤抑制能力,克服了传统线粒体靶向阳离子 TPP+ 在体内应用时因快速清除而带来的不确定性,甚至还能诱导与免疫疗法相关的免疫原性细胞死亡。这种新颖的策略为药物共轭系统的开发开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
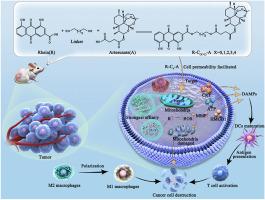
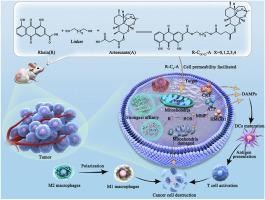
Mitochondria-targeting artesunate-rhein conjugates: Linker-modulated cell-permeability, heme-affinity and anticancer activity
Heme, abundant in the mitochondria of cancer cells, is a key target for the anticancer activity of artemisinin (ART). Current strategies to enhance the anticancer activity of ART focus solely on its delivery to heme-enriched subcellular localizations while overlooking the decisive effects of ART-heme interactions. Here, we propose an ingenious strategy that synergizes mitochondria-targeted drug delivery and linker-mediated drug conformation modulation, thereby significantly enhancing the anticancer activity of ART. By strategically conjugating artemisinin (ART) with the mitochondria-targeting rhein (R) using different linkers, we aimed to precisely adjust the conformation of the conjugates. Comprehensive computational analysis revealed that the conjugate with the optimal linker length (C4) displayed a favorable conformation that facilitated cell permeability and exhibited the highest binding affinity to heme and Fe ions. Moreover, it exhibited superior tumor suppression capabilities both in vitro and in vivo, overcoming the uncertainty of in vivo application caused by the rapid clearance of the conventional mitochondria-targeted cation TPP+, and even inducing immunogenic cell death associated with immunotherapy. This novel strategy opens up a new avenue for the development of drug conjugate systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


