在 Ni-MOF 中协同加入反应活化剂和反应促进剂,实现尿素氧化反应的超常规性能
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
最近,电化学尿素氧化反应(UOR)已成为基于尿素的能源转换商业化所需的技术。然而,由于阈值电压的能量负担和涉及六电子转移机制的反应动力学缓慢,这一新生想法受到了限制。本文首次提出了同时包含尿素活化剂和尿素促进剂的电催化剂工程。我们合成了掺氮碳装饰镍基金属有机框架(MOF)作为基础催化剂材料。在 MOF 上附着不同负载量的 MoO2 和 rGO,以获得所需的 MoO2/Ni-MOF/rGO 异质结构,并在材料中加入缺陷和晶体应变。研究表明,晶格应变和原子缺陷促进了大量 Ni3+ 活性位点的形成。优化后的样品表现出非凡的 UOR 性能,相对于 RHE 的电位值低至 1.32 V,电流密度达到 10 mA cm-2,塔菲尔斜率仅为 31 mV dec-1,反映出非常快的反应动力学。在这里,MoO2 起到了 UOR 激活剂的作用,而 rGO 的优化负载则提高了反应速度。这项工作从实验和理论两方面提出了增强电催化尿素氧化反应的新见解,为基于尿素的能量收集技术开辟了一条途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
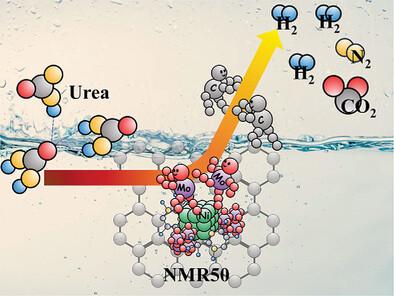
Synergistic Inclusion of Reaction Activator and Reaction Accelerator to Ni‐MOF Toward Extra‐Ordinary Performance of Urea Oxidation Reaction
Recently electrochemical urea oxidation reaction (UOR) has emerged as the technology of demand for commercialization of urea‐based energy conversion. However, the nascent idea is limited by the energy burden of threshold voltage and the sluggish reaction kinetics involving a six‐electron transfer mechanism. Herein, for the first time, the engineering of electrocatalysts are proposed with simultaneous inclusion of UOR activator and UOR accelerator. Nitrogen‐doped carbon‐decorated Ni‐based Metal Organic Framework (MOF) has been synthesized as the base catalyst material. MoO2 and rGO with varied loading have been attached to the MOF to get the desired MoO2 /Ni‐MOF/rGO heterostructure incorporating defects and crystal strain within the materials. Investigations reveal that the invoked lattice strain and atomic defects promote plenteous Ni3+ active sites. The optimized sample demonstrates extraordinary performance of UOR having the potential value as low as 1.32 V versus RHE to reach the current density of 10 mA cm−2 and the tafel slope is only 31 mV dec−1 reflecting very fast reaction kinetics. Here MoO2 plays the role of UOR activator whereas optimized loading of rGO proliferates the reaction speed. This work, experimentally and theoretically, presents a new insight to enhance electrocatalytic urea oxidation reaction opening an avenue of urea‐based energy‐harvesting technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


