基于 siRNA 的纳米治疗方法用于类风湿性关节炎的靶向给药。
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-11-19
DOI:10.1016/j.bioadv.2024.214120
引用次数: 0
摘要
类风湿性关节炎(RA)是一种全身性自身免疫性疾病,主要导致关节和组织严重受损,影响着全球数百万人。由于使用非特异性免疫抑制剂的传统 RA 治疗方法存在潜在的副作用和不同的反应,因此人们正在探索现代治疗方法。小干扰 RNA(siRNA)的研究显示了治疗 RA 的潜力。这些基于 siRNA 的疗法可包括编码 TNF-α、IL-1 和 IL-6 等促炎细胞因子的基因,以及 RANK、p38 MAPK、TGF-β、Wnt/Fz 复合物和 HIF 等其他分子靶点。通过下调这些基因的表达,基于 siRNA 的纳米制剂可减轻炎症反应、抑制免疫系统失调并防止与 RA 相关的组织损伤。通过纳米载体递送 siRNA 分子的策略可以有针对性地有效治疗风湿性关节炎,选择性地沉默与这种自身免疫性疾病病理相关的特定基因。此外,同时靶向多种分子通路可能会产生协同治疗效果,从而为 RA 患者带来更有效、更安全的治疗策略。本综述重点介绍了 RA 的深入病理、RNA 干扰介导的分子靶点、基于纳米载体的 siRNA 递送策略,以及未来解决方案所面临的挑战和机遇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
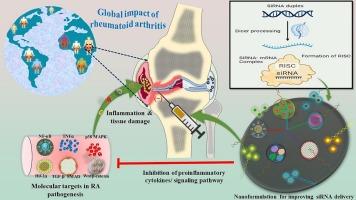
siRNA-based nanotherapeutic approaches for targeted delivery in rheumatoid arthritis
Rheumatoid arthritis (RA), characterized as a systemic autoimmune ailment, predominantly results in substantial joint and tissue damage, affecting millions of individuals globally. Modern treatment modalities are being explored as the traditional RA therapy with non-specific immunosuppressive drugs showcased potential side effects and variable responses. Research potential with small interfering RNA (siRNA) depicted potential in the treatment of RA. These siRNA-based therapies could include genes encoding pro-inflammatory cytokines like TNF-α, IL-1, and IL-6, as well as other molecular targets such as RANK, p38 MAPK, TGF-β, Wnt/Fz complex, and HIF. By downregulating the expression of these genes, siRNA-based nanoformulations can attenuate inflammation, inhibit immune system dysregulation, and prevent tissue damage associated with RA. Strategies of delivering siRNA molecules through nanocarriers could be targeted to treat RA effectively, where specific genes associated with this autoimmune disease pathology can be selectively silenced. Additionally, simultaneous targeting of multiple molecular pathways may offer synergistic therapeutic benefits, potentially leading to more effective and safer therapeutic strategies for RA patients. This review critically highlights the in-depth pathology of RA, RNA interference-mediated molecular targets, and nanocarrier-based siRNA delivery strategies, along with the challenges and opportunities to harbor future solutions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


