生物素化铂(IV)共轭氧化石墨烯纳米粒子用于乳腺癌的靶向化疗-光热联合疗法。
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-11-19
DOI:10.1016/j.bioadv.2024.214121
引用次数: 0
摘要
氧化石墨烯(GO)和基于 GO 的纳米复合材料具有优异的近红外光学吸收能力和高比表面积,因此在药物输送和光热治疗方面大有可为。在这项研究中,我们将奥沙利铂(IV)原药、PEG 化氧化石墨烯和 PEG 化生物素(PB)有效地共轭在一个平台上,用于乳腺癌治疗。该平台展示了靶向给药和在近红外激光照射下协同应用光热化疗的广阔前景。所制备的纳米复合材料(GO(OX)PB (1/1/0.2) NPs)具有超大比表面积、最小粒径(195.7 nm)、特异性靶向能力、高载药量(43.56 %)和夹带效率(89.48 %),并且在近红外激光照射(808 nm)下表现出优异的光热转换效率和光稳定性。研究人员利用人体乳腺癌细胞(MCF-7)、小鼠乳腺腺癌细胞(4T1)和 4T1-Luc 肿瘤小鼠模型对其治疗效果进行了体内外评估。研究结果表明,GO(OX)PB(1/1/0.2)NPs(+L)具有显著的细胞毒性、G2/M 期细胞周期停滞、ROS 生成、线粒体膜去极化、细胞凋亡和光热效应。与游离 OX、GO(OX)PEG (1/1/0.2) NPs (±L)和 GO(OX)PB (1/1/0.2) NPs (-L) 相比,细胞死亡的比例更高。对 4T1-Luc 肿瘤小鼠进行的体内治疗研究表明,GO(OX)PB (1/1/0.2) NPs (+L) 组合可使肿瘤完全消失,无肿瘤复发,延长生存期,减少肺转移,减轻肾毒性。血清和血液分析表明,GO(OX)PB (1/1/0.2) NPs 的全身毒性极小。在这种情况下,所开发的纳米平台可作为一种潜在的纳米药物,通过结合化疗和光热疗法来解决乳腺癌的传统肾毒性问题并防止转移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
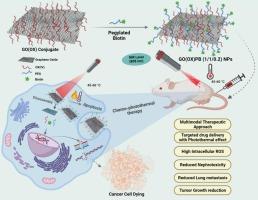
Biotinylated platinum(IV)-conjugated graphene oxide nanoparticles for targeted chemo-photothermal combination therapy in breast cancer
Graphene oxide (GO) and GO-based nanocomposites are promising in drug delivery and photothermal therapy due to their exceptional near-infrared optical absorption and high specific surface area. In this study, we have effectively conjugated an oxaliplatin (IV) prodrug, PEGylated graphene oxide, and PEGylated biotin (PB) in a single platform for breast cancer treatment. This platform demonstrates promising prospects for targeted drug delivery and the synergistic application of photothermal-chemotherapy when exposed to NIR-laser irradiation. The resulting nanocomposite (GO(OX)PB (1/1/0.2) NPs) displayed an exceptionally large surface area, minimal particle size (195.7 nm), specific targeting capabilities, a high drug load capacity (43.56 %) and entrapment efficiency (89.48 %) and exhibit excellent photothermal conversion efficiency and photostability when exposed to NIR-laser irradiation (808 nm). The therapeutic effectiveness was assessed both in vitro and in vivo conditions employing human breast cancer cells (MCF-7), mouse mammary gland adenocarcinoma cells (4T1), and 4T1-Luc tumor-bearing mouse models. The findings demonstrated that GO(OX)PB (1/1/0.2) NPs (+L) were highly effective in causing significant cytotoxicity, G2/M phase cell cycle arrest, ROS generation, mitochondrial membrane depolarization, apoptosis, and photothermal effect. This resulted in a greater percentage of cell death compared to free OX, GO(OX)PEG (1/1/0.2) NPs (±L), and GO(OX)PB (1/1/0.2) NPs (−L). The in vivo therapeutic studies on 4T1-Luc tumor-bearing mice revealed that a combination of GO(OX)PB (1/1/0.2) NPs (+L) caused complete disappearance of the tumor, no tumor recurrence, prolonged survival, reduced lung metastasis, and mitigated nephrotoxicity. The serum and blood analysis demonstrated minimal systemic toxicity of GO(OX)PB (1/1/0.2) NPs. The developed nanoplatform, in this context, may serve as a potential nanomedicine to address conventional nephrotoxicity in breast cancer and prevent metastasis by combining chemo-photothermal therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


