Nα-末端乙酰化通过 N-去甲肾上腺素途径与铁凋亡相遇
IF 6.5
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
细胞死亡可通过程序性自毁、内部破坏、意外损伤或免疫细胞介导的杀伤而发生(Green,2024 年)。其中,铁变态反应是一种受调控的破坏型细胞死亡,由营养、氧化还原和脂质代谢失衡导致的过度脂质过氧化(主要涉及铁)引发(Berndt 等人,2024 年;Dixon 等人,2012 年;Green,2024 年)。在轻度脂质过氧化的情况下,抗铁锈色素沉着作用因子可激活抗氧化防御系统,修复细胞损伤或维持氧化还原平衡。然而,当脂质过氧化超过临界阈值时,促铁蛋白沉积效应因子会更有效地转向促进铁蛋白沉积(Berndt 等人,2024 年)。此外,在临界点,细胞很可能同时激活存活和铁凋亡途径,暂时抵制铁凋亡,维持铁凋亡和存活之间的可变状态。我们最近对Na-末端(Nt-)乙酰化介导的蛋白质降解系统(Ac/N-degron途径)的研究表明了一种可信的机制,它能同时管理这些对立的促铁蛋白沉降和抗铁蛋白沉降效应因子,从而可能影响细胞的命运(图1)(Yang等人,2024年)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
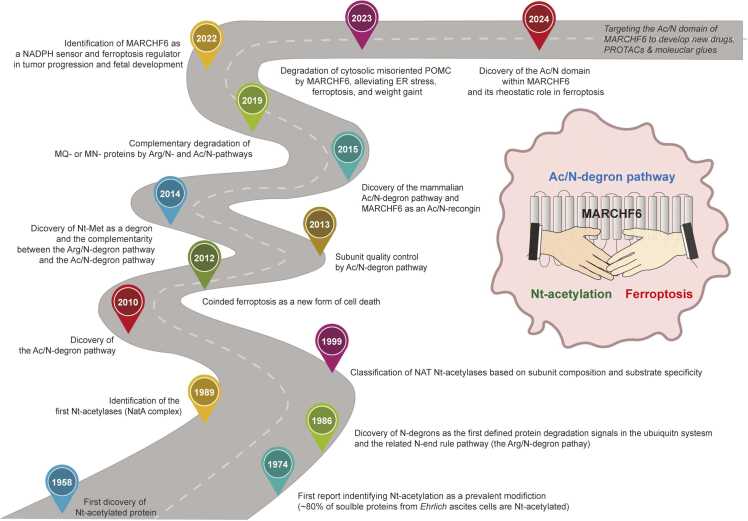
Nα-terminal acetylation meets ferroptosis via N-degron pathway
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Molecules and Cells
生物-生化与分子生物学
CiteScore
6.60
自引率
10.50%
发文量
83
审稿时长
2.3 months
期刊介绍:
Molecules and Cells is an international on-line open-access journal devoted to the advancement and dissemination of fundamental knowledge in molecular and cellular biology. It was launched in 1990 and ISO abbreviation is "Mol. Cells". Reports on a broad range of topics of general interest to molecular and cell biologists are published. It is published on the last day of each month by the Korean Society for Molecular and Cellular Biology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


