5-thio-α-d-galactopyranoside-Jacalin 与甲基 5-thio-α-d-galactopyranoside 的相互作用中的熵焓补偿。
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
合成了甲基 5-硫代-α-d-吡喃半乳糖苷,并发现它与贾卡林的结合焓比甲基 α-d-吡喃半乳糖苷更有利,这归因于硫-π 比氧-π 相互作用更大。然而,由于需要限制更灵活的硫糖,这种焓的增加被较低的结合熵所抵消,从而突出了设计有效糖模拟物固有的复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
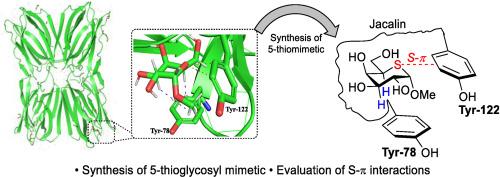
Entropy-enthalpy compensation in the methyl 5-thio-α-d-galactopyranoside–Jacalin interaction
Methyl 5-thio-α-d-galactopyranoside was synthesized and found to have a more favorable enthalpy of binding to Jacalin than methyl α-d-galactopyranoside, which is attributed to the greater magnitude of sulfur-π over oxygen-π interactions. This increase in enthalpy, however, was offset by a less favorable entropy of binding, arising from the need to constrain the more flexible thiosugar, thereby highlighting the complexities inherent in the design of effective sugar mimetics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


