NiFe2O4/g- C3N4 改性壳聚糖席夫碱复合材料用于高效去除水生环境中的铜(II)和汞(II)离子及其抗菌特性。
IF 8.5
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-11-20
DOI:10.1016/j.ijbiomac.2024.137920
引用次数: 0
摘要
通过溶胶-凝胶法,壳聚糖与 4-硝基苯甲醛发生反应,形成希夫碱,从而实现了对壳聚糖的改性。通过水热法合成了一种新型磁性氮化石墨碳/壳聚糖-席夫碱/NiFe2O3(SBIV@NiFe/g-C3N4)吸附剂,用于吸附水生环境中的铜(II)和汞(II)离子。利用红外光谱(FT-IR)、X 射线衍射(XRD)、扫描电子显微镜(SEM)、振动样品磁力计(VSM)和布鲁诺-艾美特-泰勒(BET)对合成的 SBIV@NiFe/g-C3N4 进行了表征,其表面积约为 13.657 m2/g。结果表明,磁性 SBIV@NiFe/g-C3N4 可以有效合成。研究了 pH 溶液、接触时间、金属离子浓度、吸附剂用量和共存离子等重要变量对铜(II)和汞(II)吸附的影响。在理想情况下,Cu(II) 和 Hg(II) 离子的最佳吸附容量分别为 889.76 mg/g 和 703.21 mg/g。此外,SBIV@NiFe/g-C3N4 材料还表现出简单分离的有利特性,即使经过多次重复使用,也能继续保持对铜(II)和汞(II)等重金属的高去除率。总之,使用 SBIV@NiFe/g-C3N4 这种新型吸附剂可以在短期内有效去除不同水源中的铜(II)和汞(II)。合成的 SBIV@NiFe/g-C3N4 对金黄色葡萄球菌和寻常癣菌等高致死性细菌具有更好的抗菌活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
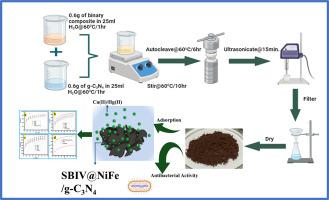
NiFe2O4/g- C3N4 modified chitosan Schiff base composite for efficient removal of Cu(II) and Hg(II) ions from the aquatic environment and its antibacterial properties
Modification of chitosan has been achieved by the reaction of chitosan with 4- nitro-benzaldehyde via the sol-gel method, resulting in a Schiff base. A novel magnetic Graphitic Carbon Nitride/chitosan-Schiff base/NiFe2O3 (SBIV@NiFe/g-C3N4) adsorbent was synthesized by hydrothermal route for the adsorption of Cu(II) and Hg(II) ions from the aquatic environment. The synthesized SBIV@NiFe/g-C3N4 was characterized using infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), vibrating sample magnetometer (VSM), and Brunauer-Emmett-Teller (BET), with a surface area of approximately 13.657 m2/g. It was anticipated by the results that magnetic SBIV@NiFe/g-C3N4 would be effectively synthesized. On Cu(II) and Hg(II) adsorption, the impacts of significant variables, including pH solution, contact duration, metal ion concentration, adsorbent dosage, and co-existing ions, were examined. Under ideal circumstances, the optimum adsorption capacities of Cu(II) and Hg(II) ions were 889.76 mg/g and 703.21 mg/g, respectively. Furthermore, the SBIV@NiFe/g-C3N4 material exhibited the beneficial property of simple separation, permitting the continuation of high removal effectiveness for heavy metals like Cu (II) and Hg(II) despite experiencing many reuse cycles. In summary, there are a lot of opportunities for the effective elimination of Cu (II) and Hg (II) from different water sources shortly with the use of SBIV@NiFe/g-C3N4, a new adsorbent. The as-synthesized SBIV@NiFe/g-C3N4 displayed better antibacterial activity against highly lethal bacteria like S. aureus and P. vulgaris.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


