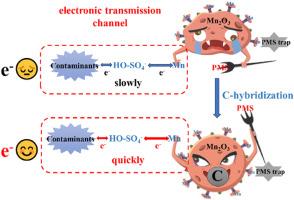
通过 C-杂化增加 Mn2O3 的路易斯酸性位点并促进电子传递,从而改善过一硫酸盐活化降解双酚 A 的效果。
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
氧化锰催化剂的表面酸度和电子传递性能对其过氧化单硫酸盐(PMS)活化性能有很大影响。本研究采用沉淀法制备了 Mn2O3 催化剂。以草酸二钠为沉淀剂制备的 C 杂化 Mn2O3-D (Mn2O3-D)催化剂表面具有更多的 Mn3+ 和路易斯酸位点,促进了 PMS 在催化剂表面的结合,在诱导 PMS 活化降解双酚 A(BPA)方面表现最佳。淬灭实验和原位电子自旋共振(ESR)结果表明,自由基和单线态氧不是高级氧化过程中的主要活性氧(ROS)。苯甲基砜(PMSO)的化学探针实验表明,PMS 与催化剂表面锰位点结合形成的≡Mn-OOSO3-逸散中间体是降解污染物的重要活性物种。污染物与催化剂表面的中间产物结合形成电子转移通道,通过氧原子转移途径和单电子转移途径直接降解。而在这一过程中,C 的杂化促进了电子转移。该研究进一步阐明了锰氧化物活化 PMS 的反应机理,为设计锰氧化物催化剂高效活化 PMS 提出了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Increasing Lewis acidic sites and promoting electron transfer of Mn2O3 by C-hybridization to improve the peroxymonosulfate activation for Bisphenol A degradation
The surface acidity and electron transfer performance of manganese oxide catalysts significantly affected its performance of peroxymonosulfate (PMS) activation. In this work, Mn2O3 catalyst was prepared by the precipitation method. The C-hybridization Mn2O3 (Mn2O3-D) catalyst prepared with disodium oxalate as a precipitant had more Mn3+ and Lewis acid sites on the surface, promoting the binding of PMS on the catalyst surface, which exhibited the best performance in inducing PMS activation to degrade bisphenol A (BPA). Quenching experiments and in situ electron spin resonance (ESR) results indicated that radicals and singlet oxygen were not the main reactive oxygen species (ROSs) in the advanced oxidation process. The chemical probe experiment of phenylmethylsulfone (PMSO) showed that the ≡ Mn-OOSO3- metastable intermediate formed by the binding of PMS with Mn sites on the catalyst surface was important active species for contaminants degradation. Contaminants combined with intermediates on the catalyst surface to form the electron transfer channels, which were directly degraded through oxygen-atom-transfer pathway and single-electron-transfer pathway. And the hybridization of C promoted the electron transfer during this process. This work further elucidated the reaction mechanism of PMS activation by manganese oxides, and proposed new ideas for the design of MnOx catalysts for efficient activation of PMS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


