调节不同原子之间形成卤素键的竞争关系
IF 2.7
2区 化学
Q3 CHEMISTRY, PHYSICAL
The Journal of Physical Chemistry A
Pub Date : 2024-11-11
DOI:10.1021/acs.jpca.4c0648310.1021/acs.jpca.4c06483
引用次数: 0
摘要
I 原子和 Br 原子分别位于一个正丁基的两端,每个原子都可以与 NH3 形成一个卤素键 (XB)。DFT 计算表明,通过在烷基链上适当放置取代基,可以逆转亲核体对较重的 I 原子而不是 Br 原子的固有偏好。烷基链上的 NH2 和 OH 基团也发生了类似的逆转,在亲电 ICCH 的 XB 中,取代基使 O 成为比 N 更好的电子供体。当一对竞争原子位于芳香环上或芳香环内时,芳香环的高流动性 π 电子云使得这种反转变得更加困难。本文章由计算机程序翻译,如有差异,请以英文原文为准。
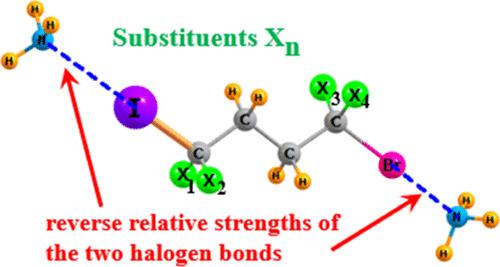
Modulating the Competition between Different Atoms to Form Halogen Bonds
I and Br atoms are placed on opposite ends of a n-butyl group, with each allowed to form a halogen bond (XB) with NH3. DFT calculations show that the intrinsic preference of the nucleophile for the heavier I over Br can be reversed by the proper placement of substituents on the alkyl chain. A similar reversal occurs for NH2 and OH groups on the alkyl chain, where substituents make the O a better electron donor than N in an XB to an electrophilic ICCH. The highly mobile π-electron cloud of an aromatic ring makes such reversals much more difficult when the pair of competing atoms are placed on, or within, such a ring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry A
化学-物理:原子、分子和化学物理
CiteScore
5.20
自引率
10.30%
发文量
922
审稿时长
1.3 months
期刊介绍:
The Journal of Physical Chemistry A is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


