母水蚤接触抗抑郁药舍曲林会导致蜕皮障碍、多代生殖和血清素能障碍
IF 4.1
2区 环境科学与生态学
Q1 MARINE & FRESHWATER BIOLOGY
引用次数: 0
摘要
舍曲林是最常用的抗抑郁药物之一,在过去几十年中,其使用量呈逐步上升趋势,而 COVID-19 的流行则加剧了这一趋势。本研究评估了舍曲林对水生微型甲壳动物大型水蚤(一种孤雌生殖的模式物种)的跨代影响。亲本大型蚤(G0)暴露于环境相关浓度的舍曲林(0.1 和 10 μg/L)中 21 天,观察暴露在个体和种群水平上引发的特异性繁殖力增加和非同步蜕皮。这些改变部分遗传给随后的三个非暴露世代(G1、G2 和 G3),表现为 G1 后代繁殖力增加和蜕皮紊乱,G2 后代繁殖力降低,G3 后代体型缩小。与蜕皮相关的基因 neverland 1 和激素受体 3 只有在暴露的一代中才同时与对照组有显著差异,这很可能是造成蜕皮不同步的原因。卵黄素在生殖过程中起着重要作用,我们的研究结果表明,卵黄素的异常表达一直持续到G3,这与舍曲林的药物靶点--5-羟色胺转运体的表达高度相关。这一结果表明,舍曲林具有持续的生殖毒性和破坏潜力,可能与血清素转运体的代偿反馈引起的血清素失调有关。舍曲林与雄性个体的出生以及双倍体素和卵黄素的上调结合在一起,被认为会通过增加生殖输入引发一种被称为 "弃船 "的自卫反应。然而,在每一代的个体繁殖试验中均未发现雄性个体,这可能表明舍曲林与种群密度之间存在某种相互作用。我们的研究结果表明,舍曲林的毒性作用可以转移到未暴露的世代,甚至产生不同的不良后果,这意味着未来的研究需要关注跨代延迟效应及其内在机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
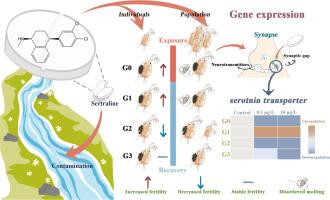
Maternal Daphnia magna exposure to the antidepressant sertraline causes molting disorder, multi-generational reproductive and serotonergic dysfunction
Sertraline, one of the most commonly used antidepressants, has exhibited a progressively escalating trend in usage over the course of the last decades years, which have been exacerbated by the COVID-19 pandemic. Here, this study assessed the transgenerational effects of sertraline on the aquatic microcrustacean Daphnia magna, a parthenogenetic model species. The parental D. magna (G0) were exposed to environmentally relevant concentrations of sertraline (0.1 and 10 μg/L) for 21 days at individual and population level, and observed exposure triggered specific increased fecundity and desynchronized molting. These alterations were partially inherited through three subsequent non-exposed generations (G1, G2, and G3), as evidenced by increased fecundity and disordered molting in G1, reduced fecundity in G2, and reduced body size of G3-offspring. The molt-related genes neverland 1 and hormone receptor 3 were significantly different to the control group simultaneously only in the exposed generation, which may well be responsible for the molting asynchrony. Vitellogenin plays an important role in reproduction, and our results indicate that its abnormal expression persists up to G3, which was highly correlated with the expression of serotonin transporter, the drug target of sertraline. This finding suggested that sertraline possesses a sustained reproductive toxicity and disrupting potential and may be associated with serotonin dysregulation caused by compensatory feedback of serotonin transporter. In combination with male birth and upregulation of doublesex and vitellogenin, sertraline was deemed to trigger a self-defense response of D. magna, known as “abandon-ship” by increasing reproductive inputs. However, no males was found in individual reproduction test in each generation, which may suggest some interaction between sertraline and population density. Our findings emphasize that the toxic effects of sertraline can be transferred to unexposed generations, even with different adverse consequences, implying that future studies need to focus on transgenerational delayed effects and the underlying mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Aquatic Toxicology
环境科学-毒理学
CiteScore
7.10
自引率
4.40%
发文量
250
审稿时长
56 days
期刊介绍:
Aquatic Toxicology publishes significant contributions that increase the understanding of the impact of harmful substances (including natural and synthetic chemicals) on aquatic organisms and ecosystems.
Aquatic Toxicology considers both laboratory and field studies with a focus on marine/ freshwater environments. We strive to attract high quality original scientific papers, critical reviews and expert opinion papers in the following areas: Effects of harmful substances on molecular, cellular, sub-organismal, organismal, population, community, and ecosystem level; Toxic Mechanisms; Genetic disturbances, transgenerational effects, behavioral and adaptive responses; Impacts of harmful substances on structure, function of and services provided by aquatic ecosystems; Mixture toxicity assessment; Statistical approaches to predict exposure to and hazards of contaminants
The journal also considers manuscripts in other areas, such as the development of innovative concepts, approaches, and methodologies, which promote the wider application of toxicological datasets to the protection of aquatic environments and inform ecological risk assessments and decision making by relevant authorities.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


