生物学中的耦合双核铜位点:实验校准计算视角
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
一大类 O2 激活耦合双核铜(CBC)金属酶包含一个独特的[Cu2O2]催化核心。该核心负责催化具有挑战性的生化转化,特别是取代酚的区域选择性单氧化/氧化。尽管近四十年来进行了大量的实验和理论研究,但人们对 CBC 酶的各种反应性的影响因素仍然只有部分了解。在这篇综述中,我们重点介绍了光谱学、动力学实验和最先进计算(包括混合量子力学和分子力学 QM/MM 以及先进的波函数理论 WFT 方法)之间的最新协同作用,这些协同作用为 CBC 酶催化的酚类底物正羟基化的初始阶段提供了确凿的机理图景。我们强调在中间产物的光谱和动力学实验数据的支持下,经过校准的理论计算能够提供对催化反应坐标的确切见解。我们对之前为阐明四类 CBC 蛋白(血蓝蛋白、儿茶酚氧化酶、酪氨酸酶、邻氨基苯酚加氧酶)的结构-功能相关性所做的努力进行了批判性回顾。我们概述了如何通过对不同 CBC 酶类的系统机理理解来揭示其难以捉摸的结构-功能相关性,从而为利用 [Cu2O2] 催化核心在原生生物环境之外的材料和生物催化应用开辟新的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
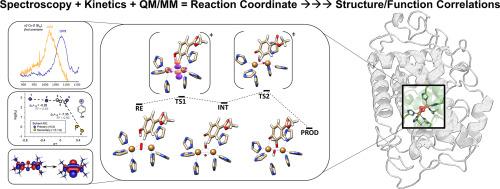
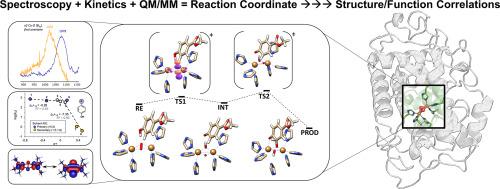
Coupled binuclear copper sites in biology: An experimentally-calibrated computational perspective
The broad class of O2-activating coupled-binuclear copper (CBC) metalloenzymes contain a unique [Cu2O2] catalytic core. This core is responsible for catalyzing challenging biochemical transformations, particularly the regioselective monooxygenations/oxidations of substituted phenols. Despite almost four decades of intense experimental and theoretical research, the factors governing the diverse reactivity of CBC enzymes had remained only partially understood. In this review, we highlight the recent synergy between spectroscopy, kinetic experiments, and state-of-the-art computations (including hybrid quantum and molecular mechanical, QM/MM, and advanced wave function theory, WFT, methods) that provided a conclusive mechanistic picture of the initial stages of the ortho-hydroxylation of phenolic substrates catalyzed by the CBC enzyme tyrosinase (Ty). We emphasize the power of calibrated theoretical calculations, supported by experimental spectroscopic and kinetic data on intermediates, in providing definitive insight into the catalytic reaction coordinate. We provide a critical review of previous efforts towards elucidating structure-function correlations over the four CBC protein classes (hemocyanins, catechol oxidases, tyrosinases, o-aminophenol oxygenases). We outline how a systematic mechanistic understanding across the different CBC enzyme classes could uncover their elusive structure-function correlations, opening new possibilities for utilizing the [Cu2O2] catalytic core outside its native biological context for applications in materials and biocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


