在掺杂 P 的石墨烯上锚定 Ru-Ru2P 异质结以增强水电解的 HER 性能
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水电解中的氢进化反应(HER)需要高活性和高稳定性的电催化剂。本研究采用温和的熔盐模板法,在掺磷石墨烯(PCSG)上锚定了钌-磷化钌异质结(Ru-Ru2P)。石墨烯由海南废弃的椰子壳合成。我们发现,异质结结构可以加速电子转移,而石墨烯则提供了更多暴露的活性位点,从而显著提高了催化剂的 HER 活性。用 30 毫克 RuCl3 在 800 °C 下制备的催化剂(Ru-Ru2P/2D-PCSG-800)在 10 mA cm-2 的电流密度下,在碱性电解质中和酸性电解质中分别表现出 31 mV 和 57 mV 的低 HER 过电位,与商业 20% Pt/C 催化剂(在碱性电解质中和酸性电解质中分别为 36 mV 和 40 mV)相当。此外,该催化剂具有很高的稳定性,在运行 125 小时后电流密度没有发生显著变化。密度泛函理论计算表明,Ru-Ru2P 异质结可以重排电荷并改变 Ru d 带中心,优化活性⁎H 的吸附能(|ΔG⁎H|)和⁎H-OH 键的断裂能(ΔGH2O)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
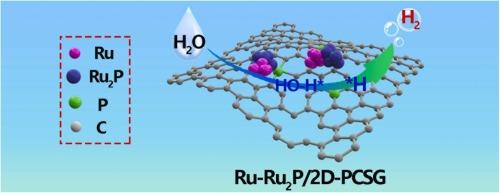
Anchoring Ru-Ru2P heterojunction on P-doped graphene for enhanced HER performances of water electrolysis
Highly active and stable electrocatalysts are highly desired for the hydrogen evolution reaction (HER) in water electrolysis. In this study, a ruthenium-ruthenium phosphide heterojunction (Ru-Ru2P) anchored on phosphorus-doped graphene (PCSG) was fabricated via a mild molten salt template method. The graphene was synthesized from discarded coconut shells sourced from Hainan. We found that the heterojunction structure could accelerate electron transfer, while the graphene provided more exposed active sites, significantly enhancing the HER activity of the catalyst. The catalyst prepared at 800 °C with 30 mg of RuCl3 (Ru-Ru2P/2D-PCSG-800) exhibited low HER overpotentials of 31 mV in alkaline and 57 mV in acidic electrolytes at a current density of 10 mA cm-2, respectively, which were comparable to those of commercial 20% Pt/C catalyst (36 mV in alkaline and 40 mV in acidic electrolytes). Moreover, the catalyst demonstrated high stability with no significant change in current density after 125 hours of operation. Density functional theory calculations revealed that the Ru-Ru2P heterojunction could rearrange charge and modify the Ru d-band center, optimizing the adsorption energy of active ⁎H (|ΔG⁎H|) and breakage energy of ⁎H-OH bond (ΔGH2O).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


