PAD 树脂:实时反馈、高效解决六价铬污染问题的智能吸附剂
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
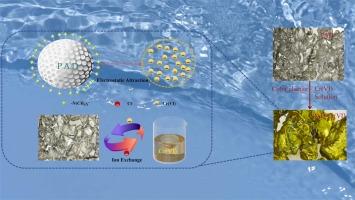
为解决六价铬污染这一紧迫问题,保护水生生态系统,我们对一种通过环保型水聚合工艺合成的聚丙烯酰胺-共甲基丙烯酰氧乙基三甲基氯化铵(PAD)树脂进行了深入研究。这种树脂不仅具有很高的六价铬去除能力,而且还采用了比色传感机制,在吸附六价铬时会从透明变为黄色,从而对修复过程进行实时、非侵入式监测和优化。根据 Langmuir 模型,在 pH 值为 4.78 和温度为 15℃的条件下,PAD 对六价铬的最大吸附容量为 135.32 mg/g。其吸附动力学符合伪一阶模型和 Langmuir 等温线,表明吸附位点均匀且存在有利的相互作用。热力学分析进一步揭示了吸附过程的自发和放热性质,使其适合在常温下大规模应用。在天然湖泊水基六价铬模拟废水中,PAD 树脂对 4.在天然湖泊水基六价铬模拟废水中,PAD 树脂对 4.82 mg/L 六价铬的去除率高达 99.54%(填充柱直径为 3 厘米,高 30 厘米;PAD 用量为 1.6 克,流速为 5 毫升/分钟,吸附时间为 60 分钟,pH 值为中性),有效地将六价铬残留浓度降至 0.022 mg/L,远低于世界卫生组织的限值(0.05 mg/L)。总之,PAD 树脂是一种创新、高性能、智能型六价铬吸附剂,它超越了传统吸附剂的局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PAD resin: An intelligent adsorbent for solving Cr(VI) pollution with real-time feedback and high efficiency
To address the urgent issue of Cr(VI) pollution and protect aquatic ecosystems, we conducted an exhaustive investigation into a Poly(acrylamide-co-methacryloyloxyethyl trimethylammonium chloride) (PAD) resin synthesized through an environmentally friendly aqueous polymerization process. This resin not only boasts a high capacity for Cr(VI) removal but also incorporates a colorimetric sensing mechanism that visually transitions from transparent to yellow upon Cr(VI) adsorption, offering real-time, non-invasive monitoring and optimization of the remediation process. According to the Langmuir model, at a pH of 4.78 and a temperature of 15 ℃, the maximum adsorption capacity of PAD for Cr (VI) is 135.32 mg/g. Its adsorption kinetics conform to a pseudo-first-order model and Langmuir isotherm, indicating uniform adsorption sites and favorable interactions. Thermodynamic analysis further reveals the spontaneous and exothermic nature of the adsorption process, making it suitable for large-scale applications at ambient temperatures.In natural lake water-based Cr(VI) simulated wastewater, PAD resin achieved a remarkable removal efficiency of 99.54 % for 4.82 mg/L Cr(VI) (The filling column had a diameter of 3 cm and a height of 30 cm; The PAD dosage was 1.6 g, with a flow rate of 5 mL/min and an adsorption time of 60 min, at a neutral pH), effectively reducing residual Cr(VI) concentrations to 0.022 mg/L, well under WHO limits (0.05 mg/L). Additionally, its 93.68 % capacity retention after four HCl regeneration cycles underscores economic feasibility & sustainability.In summary, PAD resin stands out as an innovative, high-performance, and intelligent Cr(VI) adsorbent that transcends the limitations of traditional adsorbents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


