生成 iPSC 衍生的人静脉内皮细胞,用于血管畸形建模和药物研发
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
静脉畸形(VMs)是一种常见的血管畸形,通常归因于静脉内皮细胞(VECs)内的非遗传性体细胞突变。VMs缺乏可靠的疾病模型,阻碍了药物发现。在这里,我们设计了一种稳健的方案,通过视黄醇信号通路操纵细胞周期动态,生成人类诱导 VECs(iVECs)。我们在诱导多能干细胞(iPSCs)的 TIE2 基因座中引入了 L914F 突变,结果表明,突变的 iVECs 移植到小鼠体内后会形成扩张的血管,从而再现了在血管瘤中观察到的表型特征。此外,我们还利用深度神经网络和高通量数字 RNA 与扰动基因测序(DRUG-seq)方法进行了药物筛选,并证明博舒替尼能有效挽救体外和体内的疾病表型。总之,通过利用基因组编辑和干细胞技术,我们生成了虚拟医学模型,从而能够开发出更多的疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
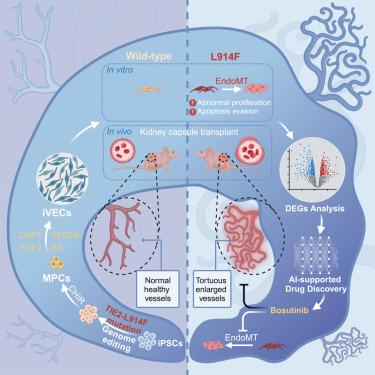
Generation of iPSC-derived human venous endothelial cells for the modeling of vascular malformations and drug discovery
Venous malformations (VMs) represent prevalent vascular anomalies typically attributed to non-inherited somatic mutations within venous endothelial cells (VECs). The lack of robust disease models for VMs impedes drug discovery. Here, we devise a robust protocol for the generation of human induced VECs (iVECs) through manipulation of cell-cycle dynamics via the retinoic signaling pathway. We introduce an L914F mutation into the TIE2 gene locus of induced pluripotent stem cells (iPSCs) and show that the mutated iVECs form dilated blood vessels after transplantation into mice, thereby recapitulating the phenotypic characteristics observed in VMs. Moreover, utilizing a deep neural network and a high-throughput digital RNA with perturbation of genes sequencing (DRUG-seq) approach, we perform drug screening and demonstrate that bosutinib effectively rescues the disease phenotype in vitro and in vivo. In summary, by leveraging genome editing and stem cell technology, we generate VM models that enable the development of additional therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


