氢键强度对明胶相分离水凝胶结构和性能的影响
IF 4.5
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
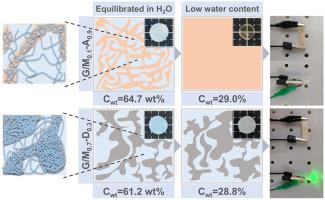
动态粘接的韧性水凝胶通常包含相分离结构,可促进多尺度能量耗散。然而,人们对粘接强度对水凝胶相分离结构和性能的影响研究不足。在这项研究中,通过改变丙烯酸共聚物中甲基的含量来调节相分离明胶/丙烯酸共聚物水凝胶中的氢键强度。研究发现,水凝胶的机械性能是由含水量和氢键强度之间的协同效应决定的。无论是弱氢键还是强氢键,都会导致水凝胶含水量高而机械强度低。据观察,随着氢键强度的增加,水凝胶中的链致密区变得更加凝结和更大。氢键强度高的凝胶含水量高,是因为形成了凝结的链致密区,从而防止了水凝胶的显著收缩。高含水量和凝结链致密区相结合,在水凝胶中形成了稀疏的链松散区,为离子传输提供了水通道,即使含水量降低到 28.9 wt%,也能实现高离子传导性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of hydrogen bonding strength on the structure and properties of phase-separated hydrogel from gelatin
Dynamic bonded tough hydrogels often contain phase-separated structures that facilitate multiscale energy dissipation. However, the influence of bonding strength on the phase-separated structures and properties of hydrogels has been understudied. In this work, the hydrogen bonding strength within phase-separated gelatin/acrylic acid copolymer hydrogels was modulated by varying the methyl group content in the acrylic acid copolymer. It was found the mechanical properties of the hydrogels are determined by a synergistic effect between water content and hydrogen bond strength. Both weak and strong hydrogen bonds resulted in the hydrogel with high water content and low mechanical strength. It was observed that the chain dense regions within the hydrogels became more condensed and larger with increasing hydrogen bonding strength. The high water content in gels with strong hydrogen bonds is attributed to the formation of condensed chain dense regions, which prevent significant shrinkage of the hydrogel. The combination of high water content and condensed chain dense regions resulted in sparse chain loose regions within the hydrogel, which provided water channels for ion transport, enabling high ionic conductivity even at a reduced water content of 28.9 wt%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


