泰国红树林 Xylocarpus moluccensis 中的十种类柠檬素和三种原柠檬素。
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从泰国红树林 Xylocarpus moluccensis 的种子中分离出了 10 种新的柠檬醛类化合物,分别命名为木犀草酮 E-N (1-10),以及两种新的原木犀草酮类化合物,分别命名为木犀草酮 O (11) 和 P (12),还有已知的化合物--丙酮庚二酮 (13)。这些化合物的结构是通过大量的核磁共振光谱数据、单晶 X 射线衍射分析和实验 ECD 光谱对比确定的。通过使用 Cu Kα 辐射进行单晶 X 射线衍射分析,明确确定了木糖醇酮 E (1) 和 L (8) 的绝对构型。木糖醇内酯 E-L(1-8)属于墨西哥内酯型柠檬酸类化合物,其中木糖醇内酯 J(6)和 K(7)含有一个 C7/C28δ 内酯环,而木糖醇内酯 M(9)和 N(10)属于葭苷型柠檬酸类化合物。木糖醇酮 O (11) 和 P (12) 是两种新的原柠檬酸类化合物。此外,还首次完整地分配了丙酮桧酮(13)的 1H 和 13C NMR 光谱数据。在生物测定中,木犀草酮 F (2)、M (9) 和 P (12) 对 LPS 诱导的 RAW 264.7 细胞产生的 NO 具有中等程度的抑制活性,IC50 值分别为 31.54 ± 7.27、62.84 ± 17.62 和 22.7 ± 6.56 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
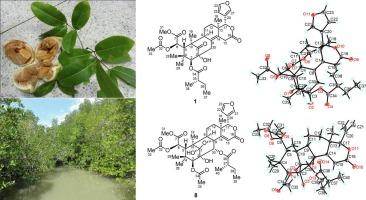
Ten limonoids and three protolimonoids from the Thai mangrove Xylocarpus moluccensis
Ten new limonoids, named xylomolones E–N (1−10), and two new protolimonoids, named xylomolones O (11) and P (12), were isolated from seeds of the Thai mangrove Xylocarpus moluccensis, together with the known compound, hispidone acetonide (13). The structures of these compounds were established by extensive NMR spectroscopic data, single-crystal X-ray diffraction analysis, and comparison of experimental ECD spectra. The absolute configurations of xylomolones E (1) and L (8) were unambiguously determined by single-crystal X-ray diffraction analyses, conducted with Cu Kα radiation. Xylomolones E–L (1–8) are mexicanolide-type limonoids, among which xylomolones J (6) and K (7) contain a C7/C28 δ-lactone ring, whereas xylomolones M (9) and N (10) are phragmalin-type limonoids. Xylomolones O (11) and P (12) are two new protolimonoids. In addition, the 1H and 13C NMR spectroscopic data for hispidone acetonide (13) was first assigned completely. In bioassay, xylomolones F (2), M (9), and P (12) exhibited moderate inhibitory activity against the production of NO in LPS-induced RAW 264.7 cells with IC50 values of 31.54 ± 7.27, 62.84 ± 17.62, and 22.7 ± 6.56 μM, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


