硒调节 Pt/Al2O3 电子结构可诱导催化 CO 氧化过程中的失活现象
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
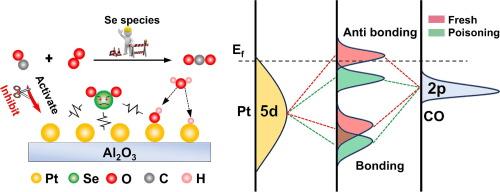
工业源(如钢铁和电力活动)继续排放大量的一氧化碳,但由于缺乏相关法规,并未受到与移动源同等程度的关注。本研究报告指出,汽车一氧化碳催化转化器中使用的商用铂/铝氧化物催化剂可能会面临来自工业源的严重硒(Se)中毒失活问题。Pt/Al2O3 催化剂(250 °C)的翻转频率 (TOF) 从 2.18 s-1 降至 0.12 s-1,而 Se 的沉积量仅为 0.49 wt% (ICP)。硒的沉积导致铂的 5d 轨道向高能态移动,从而提高了铂的氧化态。因此,铂的 5d 电子对吸附的 CO 分子的 2π* 反键轨道的反拨作用受到抑制,削弱了铂/Al2O3 与 CO 反键轨道之间的键合,从而大大降低了 CO 的活化能力。DRIFTS 结果结合表观和微观动力学表明,Se 沉积的 Pt/Al2O3 催化剂表面仍可获得 O*(500 K,θO* = 0.16),速率控制步骤从 O2 + 2* → 2O* 变为 CO + * → CO*。这项工作表明,工业源 CO 催化氧化技术的应用应充分考虑 Se 的毒性效应,但这一效应往往被忽视。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Selenium-modulated Pt/Al2O3 electronic structure induces deactivation during catalytic CO oxidation
Industrial sources, such as steel and power activities continue to emit substantial amounts of CO, but have not received the same level of attention as mobile sources due to a lack of relevant regulations. This work reports that a commercial Pt/Al2O3 catalyst used in vehicle CO catalytic converters may face severe selenium (Se) poisoning deactivation from industrial sources. The turnover frequency (TOF) of the Pt/Al2O3 catalyst (250 °C) decreased from 2.18 s−1 to 0.12 s−1 with only 0.49 wt% (ICP) Se deposition. Se deposition causes the Pt 5d orbital shift to a higher energy state, raising the oxidation state of platinum. As a result, the back-donation from the 5d electrons of Pt to the 2π* antibonding orbital of the adsorbed CO molecule is inhibited, weakening the bonding between Pt/Al2O3 and the CO antibonding orbital, thereby significantly reducing CO activation ability. DRIFTS results, combined with apparent and microscopic kinetics, indicate that the surface of Se deposited Pt/Al2O3 catalyst remains O* available (500 K, θO* = 0.16), and the rate-controlling step changes from O2 + 2* → 2O* to CO + * → CO*. The work suggests that the application of CO catalytic oxidation technology for industrial source should fully consider the toxic effect of Se, but is often neglected.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


