人工合成的 UDP-Glucose 2-epimerase(UDP-葡萄糖 2-epimerase)可通过合成酶-epimerase(合成酶-epimerase)途径实现 UDP/GDP-Mannose 的简便生产
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
尿苷/鸟苷二磷酸-甘露糖(UDP/GDP-Man)是生产含甘露糖寡糖/多糖的主要甘露糖供体。由于其合成过程复杂且成本高昂,需要多种底物和酶,因此其获取受到很大限制。天然 UDP/GDP- 葡萄糖 2-表聚酶在 UDP/GDP-Glc 和 UDP/GDP-Man 之间的 C2 表聚作用尚未报道,这是实现 UDP/GDP-Man 简便生产的主要障碍。在这里,我们创造了 UDP-Glucose 2-epimerase (Glc2E),它的行为类似于一种自然进化的酶,并在生产 UDP-Man 的过程中表现出高效的催化作用。通过重新设计核碱基识别区、移位底物隧道入口和扩大糖环旋转空间等多维工程技术,从 CDP-酪蛋白葡萄糖 2-epimerase(CDP-酪蛋白葡萄糖 2-epimerase)发展出了 Glc2E。Glc2E 能将 55.63% 的 UDP-Glc 转化为 UDP-Man,这是初始酶 stTyvE 的微量值,而且它对 GDP-Glc 二聚体的适应性从未被发现的活性发展到 23.94% 的转化率。将蔗糖合成酶与 Glc2E 联用,可以实现理论上的合成酶-表聚酶路线,利用廉价蔗糖生产 UDP/GDP-Man。在 2.5 小时内,UDP-Man 的时空产量达到最大值 8.05 克/升/小时,最终滴度为 22.54 克/升,显示了具有竞争力的应用潜力。此外,还成功合成了滴度为 3.49 克/升的 GDP-Man。我们的工作启发了催化核苷酸糖的表聚酶和糖基转移酶的酶工程。在合成酶-表聚酶途径中应用 Glc2E,为生产具有成本竞争力的甘露糖苷供体提供了一种简洁可行的合成方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
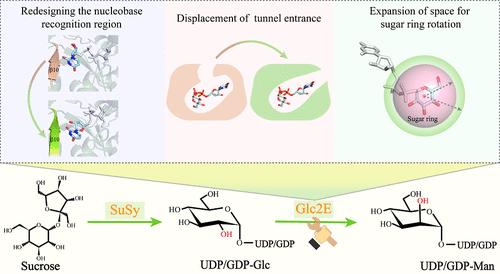
Artificially Created UDP-Glucose 2-Epimerase Enables Concise UDP/GDP-Mannose Production via the Synthase–Epimerase Route
Uridine/guanosine diphosphate-mannose (UDP/GDP-Man) is the major mannosyl donor in producing mannose-containing oligo/polysaccharides. Its acquisition is greatly limited by its complex and costly synthetic process, which requires multiple substrates and enzymes. The natural UDP/GDP-glucose 2-epimerase functioning C2 epimerization between UDP/GDP-Glc and UDP/GDP-Man remains unreported which is the main hurdle to realize concise production of UDP/GDP-Man. Here, the UDP-glucose 2-epimerase (Glc2E), which behaves like a naturally evolved enzyme, is created and exhibits high-efficient catalysis in producing UDP-Man. Multidimensional engineering, including redesigning the nucleobase recognition region, displacement of the substrate tunnel entrance, and expansion of space for sugar ring rotation, is employed to develop Glc2E from CDP-tyvelose 2-epimerase. Glc2E converts 55.63% of UDP-Glc to UDP-Man, a trace value for the initial enzyme, stTyvE, and its aptitude for GDP-Glc epimerization evolves from unobserved activity to 23.94% conversion. Coupling sucrose synthase with Glc2E achieves the theoretical synthase–epimerase route for UDP/GDP-Man production from inexpensive sucrose. The space-time-yield of UDP-Man is maximized to 8.05 g/L/h within 2.5 h, with a final titer of 22.54 g/L, demonstrating competitive application potential. Moreover, the GDP-Man is synthesized successfully at a titer of 3.49 g/L. Our work inspires the enzyme engineering for epimerases and glycosyltransferases that catalyze nucleotide sugars. The application of Glc2E in the synthase–epimerase route unlocks a concise and feasible synthetic approach for producing cost-competitive mannosyl donors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


