通过富含硫空位的剥离 MoS2 实现硝酸盐到氨的光电化学转化
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
硝酸根离子是地表水和地下水中常见的污染物。因此,在环境条件下,通过电化学和光电化学途径将其催化转化为氨气,是目前从高能耗、高碳排放的哈伯-博什工艺中生产氨气的一种有吸引力的替代方法。因此,开发具有良好耐久性和成本效益的高活性和产品选择性催化剂是非常必要的。在这项工作中,报告了剥离的 MoS2-x 作为一种高活性、高选择性的电催化剂和光电催化剂,用于将硝酸盐还原成氨。通过对块状 MoS2 进行酸处理而剥离出的 MoS2-x,只有几层厚,并且具有大量的硫空位(约 12-13%)。电化学研究和电解产物分析表明,MoS2-x 具有良好的硝酸盐还原活性。在没有光源的情况下,剥离的 MoS2-x 的法拉第效率为 69%,氨产量为 5.56 mmol gcat-1 h-1;在可见光照射下,氨产量为 7.48 mmol gcat-1 h-1,法拉第效率提高到 80%。DFT 计算支持硝酸盐和其他氮氧化物与硫空位结合,形成 *N,然后还原成氨。本文章由计算机程序翻译,如有差异,请以英文原文为准。
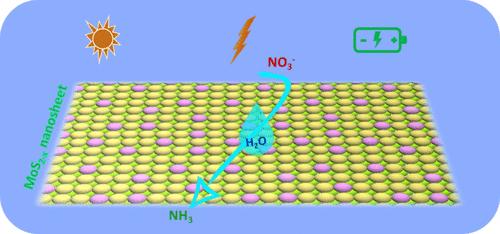
Photoenhanced Electrochemical Conversion of Nitrate to Ammonia Via Sulfur Vacancy-Rich Exfoliated MoS2
Nitrate ion is a common pollutant in surface and groundwater. Hence, its catalytic conversion into ammonia at ambient conditions by electrochemical and photoelectrochemical pathways is an attractive alternative to current ammonia production from the energy-intensive and high-carbon-featuring Haber-Bosch process. As such, developing highly active and product-selective catalysts with good durability and cost-effectiveness is highly desired. In this work, exfoliated MoS2-x is reported as a highly active and selective electrocatalyst and a photoelectrocatalyst for nitrate reduction to ammonia. Exfoliation via the acid treatment of bulk MoS2 results in exfoliated MoS2-x, which is only a few layers thick and has a high degree of sulfur vacancies (ca. 12−13%). Electrochemical studies and electrolysis product analysis reveal promising nitrate reduction activity, which is found to be highly enhanced by the application of visible light illumination. The exfoliated MoS2-x achieves a Faradaic efficiency of 69% with an ammonia yield rate of 5.56 mmol gcat–1 h–1 in the absence of a light source, which is enhanced to 80% with an ammonia yield of 7.48 mmol gcat–1 h–1 upon visible light illumination. DFT calculations support the binding of nitrate and other NOx species to the sulfur vacancies, resulting in the formation of *N, which is then reduced to ammonia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


