在离子液体 1-羟乙基-3-甲基咪唑鎓乙酸酯上氢键催化合成噁唑烷酮
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在此,我们报告了在离子液体 1-hydroxyethyl-3-methylimidazolium acetate ([OH-EMIm][OAc])上以氢键催化 1,2-苄胺、氧化苯乙烯和碳酸二甲酯的三组分反应,在 100 °C 下以极好的收率合成各种噁唑烷酮。这表明[OH-EMIm][OAc]能通过氢键活化 1,2-苄胺和氧化苯乙烯,形成中间体 2-(苄基氨基)-1-苯基-1-醇,该中间体进一步与[OH-EMIm][OAc]活化的碳酸二甲酯反应,通过中间体甲基苄基(羟基(苯基)甲基)氨基甲酸酯的分子内环化作用生成噁唑烷酮。此外,[OH-EMIm][OAc]可重复使用五次而不会失去活性。该方案提供了一种简便而新颖的草唑烷酮路线,具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
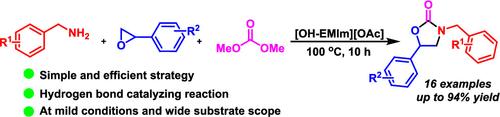
Hydrogen Bond-Catalyzed Synthesis of Oxazolidinone over Ionic Liquid 1-Hydroxyethyl-3-methylimidazolium Acetate
Herein, we report a hydrogen bonding-catalyzed three-component reaction of 1,2-benzylamine, styrene oxide, and dimethyl carbonate over ionic liquid 1-hydroxyethyl-3-methylimidazolium acetate ([OH-EMIm][OAc]) to synthesize various oxazolidinones in excellent yields at 100 °C. This indicates that [OH-EMIm][OAc] can activate 1,2-benzylamine and styrene oxide through hydrogen bonding to form intermediate 2-(benzylamino)-1-phenylethan-1-ol, which further reacts with dimethyl carbonate activated by [OH-EMIm][OAc], producing oxazolidinone via intramolecular cyclization of the intermediate methyl benzyl(hydroxy(phenyl)methyl)carbamate. Moreover, [OH-EMIm][OAc] could be reused five times without loss of activity. This protocol provides a facile and novel route to oxazolidinones, which may have promising application potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


