构建咔唑共轭双发射荧光共价有机框架,用于区分对硝基苯胺/对硝基苯酚和吸附硝基苯胺/硝基苯酚
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
硝基苯胺(NAs)和硝基苯酚(NPs)是重要的工业原料,被广泛应用于各个领域。然而,这些物质的使用会造成严重的环境污染和健康危害,因此需要对其进行紧急监测和清除,以解决环境和安全问题。NAs/NPs 异构体的存在进一步加剧了这一挑战,因此对特定异构体进行选择性分析至关重要。为此,我们合成了一种新的后修饰荧光共价有机框架(COF),称为 COF@CB,具有双发射荧光。这种合成方法是通过 "威廉姆森 "反应将高结晶度荧光 COF(COF-TTDB)与咔唑-9-乙醇(CB)耦合在一起。COF@CB 具有优异的双发射荧光、高比表面积(919.4 m2-g-1)、卓越的热稳定性和丰富的活性位点。这些特性使 COF@CB 成为一种能够同时检测和吸附的比率荧光传感器。NAs/NPs 异构体中氢键位点的不同数量和排列影响了 COF@CB 的分子内电荷转移(ICT)效应,从而使 COF@CB 比率荧光传感器能够区分并选择性地检测 p-NA/p-NP 与异构体。对实际水样的分析进一步证实了该传感器在检测 p-NA/p-NP 方面的有效性。此外,COF@CB 比率荧光传感器上存在多个活性位点,有利于吸附 NAs/NPs,从而促进将它们从实际样品中去除。本文章由计算机程序翻译,如有差异,请以英文原文为准。
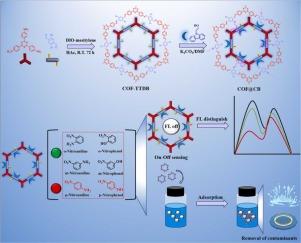
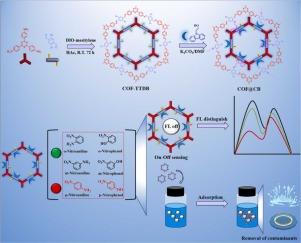
Construction of carbazole-conjugated dual-emission fluorescent covalent organic framework for distinguishing p-nitroaniline/p-nitrophenol and adsorbing nitroanilines/nitrophenols
Nitroanilines (NAs) and nitrophenols (NPs), crucial industrial raw materials, are extensively utilized across various sectors. However, the environmental pollution and health hazards stemming from their usage are significant, necessitating urgent monitoring and removal to address environmental and safety concerns. The challenge is further compounded by the presence of NAs/NPs isomers, making the selective analysis of specific isomers crucial. In response, a new post-modified fluorescent covalent organic framework (COF) termed COF@CB, exhibiting dual-emission fluorescence, was synthesized. This synthesis involved coupling a high-crystallinity fluorescent COF (COF-TTDB) with carbazole-9-ethanol (CB) via a “Williamson” reaction. COF@CB featured exceptional dual-emission fluorescence, a high specific surface area (919.4 m2·g-1), superior thermal stability, and abundant active sites. These attributes enabled COF@CB to function as a ratiometric fluorescence sensor capable of simultaneous detection and adsorption. The distinct number and arrangement of hydrogen bond sites in NAs/NPs isomers influenced the intramolecular charge transfer (ICT) effects on COF@CB, thereby enabling the COF@CB-ratiometric fluorescence sensor to distinguish and selectively detect p-NA/p-NP from isomers. Analysis of actual water samples further underscored the sensor's effectiveness in detecting p-NA/p-NP. Furthermore, the presence of multiple active sites on the COF@CB-ratiometric fluorescence sensor facilitated the adsorption of NAs/NPs, promoting the removal of them from actual samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


