铜离子靶向与有机抑制剂相结合以增强黄铁矿浮选抑制作用的机理
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究以黄铁矿为研究对象,研究了铜离子增强型有机抑制剂抑制黄铁矿浮选的行为和机理。微浮选实验表明,加入 CuSO4 和 N,N-二甲基二硫代氨基甲酸盐(N,N-DDS)后,黄铁矿在 pH 值为 8.5 时的浮选收集效率从 82.37% 下降到 21.03%。红外光谱研究表明,加入 CuSO4 会显著增强黄铁矿表面 N,N-DDS 的特征峰。X 射线光电子能谱 (XPS) 显示,加入 CuSO4 和 N,N-DDS 后,黄铁矿表面 N 1s 的相对原子浓度大大提高,其中 N 1s 来自 N,N-DDS。飞行时间二次离子质谱显示,在添加 CuSO4 前后,黄铁矿表层 CSN- 的归一化峰值分别为 0.0116 和 0.0032。上述结果都表明,Cu 离子促进了 N,N-DDS 在黄铁矿上的吸附,并促进了复合物 ((CH3)2NCSS)2Cu 的形成。-NCS中的N原子和S原子与金属离子形成化学键,这是N,N-DDS与黄铁矿相互作用的主要机制。这一过程降低了黄铁矿的可浮性,实现了对黄铁矿的选择性抑制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
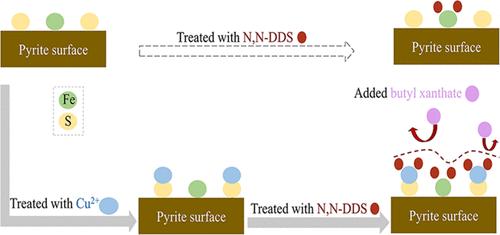
Mechanism of Cu Ion Targeting Combined with an Organic Depressant To Enhance the Flotation Depression of Pyrite
In this study, pyrite was used as the research object, and the behavior and mechanism of a copper-ion-enhanced organic depressant in depressing pyrite flotation were studied. Microflotation experiments showed that, after the addition of CuSO4 and N,N-dimethyldithiocarbamate (N,N-DDS), the floating collection efficiency of pyrite declined from 82.37 to 21.03% at pH 8.5. Infrared spectroscopy studies indicated that the addition of CuSO4 caused a significant enhancement in the characteristic N,N-DDS peaks on the pyrite surface. X-ray photoelectron spectroscopy (XPS) revealed that, following the addition of CuSO4 and N,N-DDS, the relative atomic concentration of N 1s considerably enhanced on the surface of pyrite, with N 1s derived from N,N-DDS. Time-of-flight secondary ion mass spectrometry showed that, before and after the addition of CuSO4, the normalized peak values of CSN– on the pyrite superficial layer were 0.0116 and 0.0032, respectively. All of the above-mentioned results indicate that the Cu ion promotes the adsorption of N,N-DDS on pyrite and facilitates the formation of complex ((CH3)2NCSS)2Cu. The N and S atoms in −NCS and metal ions formed chemical bonds, which were the primary mechanism of the interaction between N,N-DDS and pyrite. This process reduces the floatability of pyrite and achieves the selective depression of pyrite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


