发现 CLPP-1071 是一种具有强大体内抗肿瘤活性的特效口服人类 ClpP 激活剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
人类酪蛋白溶解蛋白酶P(ClpP)是维持线粒体蛋白质组平衡所必需的,激活它被越来越多地认为是一种有前景的癌症治疗策略。在此,我们基于结构引导药物设计,通过在处于 III 期临床试验阶段的 ClpP 激活剂 ONC201 的亚胺吡啶酮支架上引入一个甲基,发现了一系列强效 ClpP 激活剂。通过对先导化合物的结构优化,最理想的化合物 CLPP-1071 表现出了极强的 ClpP 激动活性(EC50 = 23.5 nM,比 ONC201 强 107.1 倍),并能抑制 HL60 细胞的增殖(IC50 = 4.6 nM,比 ONC201 强 169.2 倍)。CLPP-1071 具有良好的药代动力学特性,通过口服给药可有效延长小鼠 MOLM13 和 HL60 异种移植模型的寿命。CLPP-1071 是迄今为止报道的最强效、口服疗效最好的 ClpP 激活剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
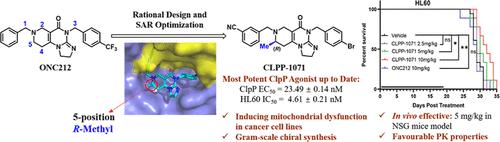
Discovery of CLPP-1071 as an Exceptionally Potent and Orally Efficacious Human ClpP Activator with Strong In Vivo Antitumor Activity
Human sapiens caseinolytic protease P (ClpP) is essential for maintaining mitochondrial proteome homeostasis, and its activation is increasingly recognized as a promising cancer therapy strategy. Herein, based on structure-guided drug design, we discovered a series of potent ClpP activators by introducing a methyl group to the imipridone scaffold of the ClpP activator ONC201 in Phase III clinical trials. Through structural optimization of the lead compound, the most optimal compound, CLPP-1071, exhibited exceptionally potent ClpP agonistic activity (EC50 = 23.5 nM, 107.1-fold stronger than ONC201) and inhibited the proliferation of HL60 cells (IC50 = 4.6 nM, 169.2-fold stronger than ONC201). CLPP-1071 possesses good pharmacokinetic properties and effectively prolongs the lifespan in the MOLM13 and HL60 xenograft models in mice through oral administration. CLPP-1071 is the most potent and orally efficacious ClpP activator reported to date.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


