Na+-V-ATP酶抑制剂抑制VRE生长并揭示Na+通路结构
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
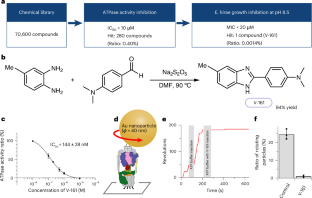
耐万古霉素肠球菌(VRE)是造成医院内感染,尤其是心内膜炎和败血症的主要原因。随着抗生素对 VRE 的疗效不断降低,迫切需要新的抗菌药物。我们之前的研究表明,Na+转运V-ATP酶在平肠球菌碱性条件下的生长中起着至关重要的作用。在本研究中,我们从 70 600 种化合物中发现了一种能明显抑制平肠球菌 V-ATP 酶活性的化合物 V-161。V-161 不仅能抑制 VRE 在碱性条件下的生长,还能显著抑制 VRE 在小鼠小肠中的定植。此外,我们还揭示了因 V-161 结合而形成的膜 VO 部分的高分辨率结构。V-161 与构成膜中 Na+ 转运途径的 c 环和 a 亚基的界面结合,从而停止了膜的旋转。这一结构见解为开发治疗 VRE 的药物提供了潜在途径,并阐明了 Na+ 转运途径和机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Na+-V-ATPase inhibitor curbs VRE growth and unveils Na+ pathway structure
Vancomycin-resistant Enterococcus faecium (VRE) is a major cause of nosocomial infections, particularly endocarditis and sepsis. With the diminishing effectiveness of antibiotics against VRE, new antimicrobial agents are urgently needed. Our previous research demonstrated the crucial role of Na+-transporting V-ATPase in Enterococcus hirae for growth under alkaline conditions. In this study, we identified a compound, V-161, from 70,600 compounds, which markedly inhibits E. hirae V-ATPase activity. V-161 not only inhibits VRE growth in alkaline conditions but also significantly suppresses VRE colonization in the mouse small intestine. Furthermore, we unveiled the high-resolution structure of the membrane VO part due to V-161 binding. V-161 binds to the interface of the c-ring and a-subunit, constituting the Na+ transport pathway in the membrane, thereby halting its rotation. This structural insight presents potential avenues for developing therapeutic agents for VRE treatment and elucidates the Na+ transport pathway and mechanism. The authors identify V-161, a compound that inhibits enterococcal Na+-V-ATPase and reduces VRE colonization in mice. The high-resolution structure of V-161 reveals its action on the Na+ transport pathway, offering new therapeutic insights.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


