应激反应能力的差异决定了李斯特菌种内毒力的异质性和宿主的适应性
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
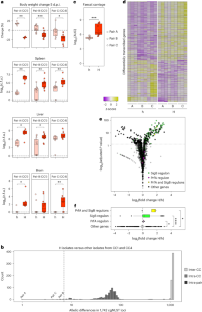
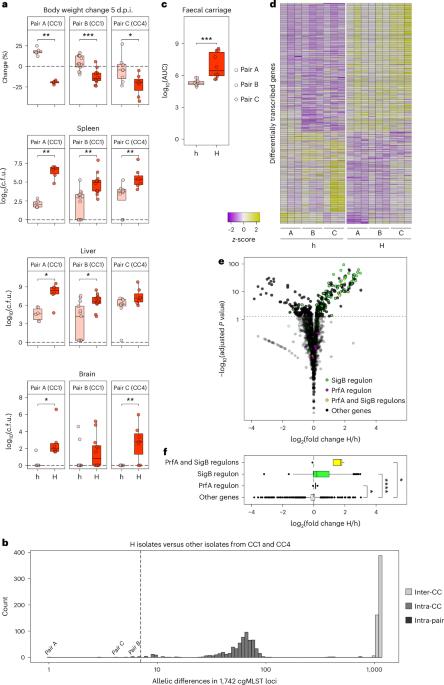
微生物致病是由毒力基因的表达介导的。然而,由于毒力基因含量相同的微生物在致病潜能方面可能存在差异,因此必须涉及其他毒力决定因素。在这里,我们通过对大量模式病原体单核细胞增生李斯特菌分离株的比较基因组学和转录组学、延时显微镜、体外进化和体内实验相结合,证明单核细胞增生李斯特菌分离株的个体应激反应性决定了它们各自在体内的毒力水平,并反映了它们对宿主的适应程度。导致单核细胞增多性乳酸杆菌毒力异质性的转录特征由应激反应调节因子 SigB 介导,并由不同的应激反应性驱动。SigB 通路响应性的调整是多基因的,受多种单独罕见基因变异的影响。这项研究揭示了微生物毒力的总体决定因素,挑战了将附属毒力基因含量作为种内毒力异质性主要决定因素的模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Differential stress responsiveness determines intraspecies virulence heterogeneity and host adaptation in Listeria monocytogenes
Microbial pathogenesis is mediated by the expression of virulence genes. However, as microbes with identical virulence gene content can differ in their pathogenic potential, other virulence determinants must be involved. Here, by combining comparative genomics and transcriptomics of a large collection of isolates of the model pathogen Listeria monocytogenes, time-lapse microscopy, in vitro evolution and in vivo experiments, we show that the individual stress responsiveness of L. monocytogenes isolates determines their respective levels of virulence in vivo and reflects their degree of host adaptation. The transcriptional signature that accounts for the heterogeneity in the virulence of L. monocytogenes species is mediated by the stress response regulator SigB and driven by differential stress responsiveness. The tuning of SigB pathway responsiveness is polygenic and influenced by multiple, individually rare gene variations. This study reveals an overarching determinant of microbial virulence, challenging the paradigm of accessory virulence gene content as the major determinant of intraspecies virulence heterogeneity. Differences in virulence across the Listeria monocytogenes species are determined by the fine-tuning of SigB pathway responsiveness and reflect host adaptation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


