脂质体介导的多西他赛增强抗肿瘤效果与 BRD4-PROTAC 作为乳腺癌化疗/免疫疗法的增效剂。
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
据报道,蛋白水解靶向嵌合体(PROTACs)能有效降解细胞内的致癌蛋白,为癌症治疗提供了一种理想的策略。ARV825是一种含溴结构域蛋白4(BRD4)-PROTAC,已证明能增强经典化疗药物多西他赛(DTX)的抗肿瘤效果。然而,ARV825 在体内的广泛应用还面临三大挑战:溶解性差、渗透性差和脱靶效应。此外,如何将 ARV825 和 DTX 有效地联合递送至肿瘤组织以产生协同治疗效果的问题仍未解决。本研究利用脂质体作为 ARV825 和 DTX 的共同给药载体,以有效解决这些问题。成熟的脂质体大大提高了 ARV825 和 DTX 的溶解度,同时在仿血条件下保持了持续释放特性。共负载脂质体通过增强的渗透性和滞留(EPR)效应在肿瘤组织中积累。内化后,ARV825 能有效降解细胞内 BRD4 蛋白,下调 Bcl-2 和 PD-L1 蛋白的表达,从而增加肿瘤细胞凋亡,增强肿瘤免疫反应。这反过来又增强了 DTX 在体内的抗肿瘤效果,而不会产生不良副作用。总之,BRD4-PROTAC 可与传统化疗药物 DTX 发挥协同作用,脂质体可作为有效的联合给药载体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
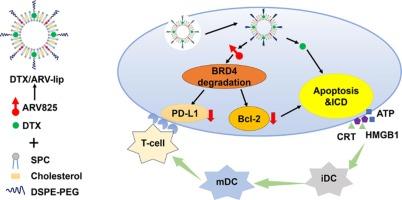
Liposomes-mediated enhanced antitumor effect of docetaxel with BRD4-PROTAC as synergist for breast cancer chemotherapy/immunotherapy
It has been reported that proteolysis-targeting chimeras (PROTACs) can effectively degrade intracellular oncogenic proteins, providing an ideal strategy for cancer treatment. ARV825, a bromodomain-containing protein 4 (BRD4)-PROTAC, has demonstrated the capacity to enhance the antitumor effect of the classic chemotherapeutic agent docetaxel (DTX). However, there are three major challenges to the broader in vivo application of ARV825: poor solubility, poor permeability, and off-target effects. Additionally, the efficient co-delivery of ARV825 and DTX to tumor tissues for a synergistic therapeutic effect remains unresolved. In this study, liposomes were utilized as co-delivery vehicles for ARV825 and DTX to effectively address these issues. The well-established liposomes significantly improved the solubility of both ARV825 and DTX while maintaining a sustained release profile in blood-mimetic conditions. The co-loaded liposomes accumulated in tumor tissues via the enhanced permeability and retention (EPR) effect. After internalization, ARV825 effectively degraded intracellular BRD4 proteins and downregulated the expression of both Bcl-2 and PD-L1 proteins, thereby increasing tumor cell apoptosis and enhancing the tumor immune response. This, in turn, augmented the antitumor effect of DTX in vivo without undesired side effects. In conclusion, BRD4-PROTAC may serve as a promising synergistic agent alongside the conventional chemotherapeutic agent DTX, with liposomes functioning as effective co-delivery vehicles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


