吡啶扩展石墨烯纳米带中优先形成的石墨氮
IF 6.2
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
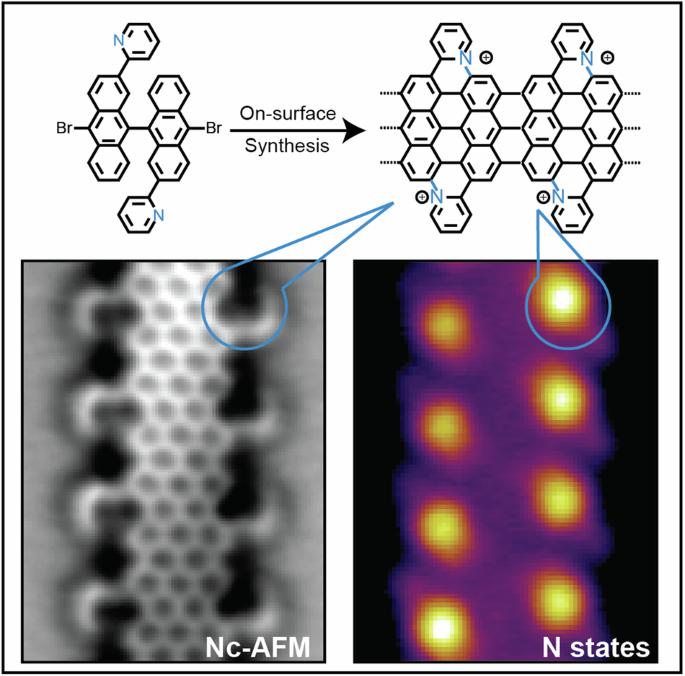
石墨烯纳米带(GNR)是纳米宽的石墨烯条带,因其量子约束产生的可调电子和磁性能而备受关注。操纵其电子特性的一种有前途的方法是用氮等杂原子取代碳,根据杂原子的位置,可预测出不同的效果。在本研究中,我们介绍了在金(111)表面通过表面合成法实现的用吡啶环扩展 7 原子宽的扶手椅石墨烯纳米带(7-AGNR)的边缘。高分辨率结构表征证实了这一目标结构,表明在生长过程中主要形成了碳-氮(C-N)键(超过 90% 的单元)。密度泛函理论(DFT)模拟阐明并证实了这种受青睐的成键途径。此外,对电子特性的分析表明,由于电荷转移到金(111)基底,并伴有氮定位态的存在,该化合物具有金属特性。我们的研究结果强调了 C-N 键在金属表面的成功形成,为设计新的 GNR 提供了启示,这种 GNR 含有置换氮原子,可以精确控制其电子特性。据预测,石墨烯纳米带中石墨氮原子的存在会强烈影响其电子特性,但在此类结构中实验形成氮原子仍然具有挑战性。在本文中,作者报告了在 Au(111) 上表面合成吡啶延伸的 7-臂对轴石墨烯纳米带的过程,通过形成 C-N 键,在完全平面化后优先形成石墨氮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Preferential graphitic-nitrogen formation in pyridine-extended graphene nanoribbons
Graphene nanoribbons (GNRs), nanometer-wide strips of graphene, have garnered significant attention due to their tunable electronic and magnetic properties arising from quantum confinement. A promising approach to manipulate their electronic characteristics involves substituting carbon with heteroatoms, such as nitrogen, with different effects predicted depending on their position. In this study, we present the extension of the edges of 7-atom-wide armchair graphene nanoribbons (7-AGNRs) with pyridine rings, achieved on a Au(111) surface via on-surface synthesis. High-resolution structural characterization confirms the targeted structure, showcasing the predominant formation of carbon-nitrogen (C-N) bonds (over 90% of the units) during growth. This favored bond formation pathway is elucidated and confirmed through density functional theory (DFT) simulations. Furthermore, an analysis of the electronic properties reveals metallic behavior due to charge transfer to the Au(111) substrate accompanied by the presence of nitrogen-localized states. Our results underscore the successful formation of C-N bonds on the metal surface, providing insights for designing new GNRs that incorporate substitutional nitrogen atoms to precisely control their electronic properties. The presence of graphitic nitrogen atoms within graphene nanoribbons has been predicted to strongly affect their electronic properties, but its experimental formation within such structures remains challenging. Here, the authors report on the on-surface synthesis of pyridine-extended 7-armchair graphene nanoribbons on Au(111), whereby graphitic nitrogen is preferentially formed after complete planarization through the formation of C–N bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


