β,β-二取代烯磺酰胺的立体选择性氨甲基化:不对称构建较难获得的环状α,α-二取代α-氨基甲基化酮亚胺
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
β,β-二取代烯磺酰胺与甲醛亚胺(由对甲苯基甲基氨基甲酸酯原位生成)发生立体选择性亲核加成反应,生成α-氨甲基化的酮亚胺,该酮亚胺具有由两个立体和电子相似的基团(例如 Me 和 Et)取代的具有挑战性的无环四元立体中心。烯磺酰胺中的 C═C 键具有明确的几何形状,加上其手性亚磺酰基提供的强烈手性诱导,确保了在形成新的 C-C 键过程中的精确立体控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
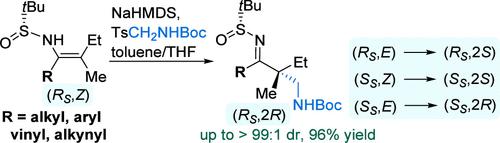
Stereoselective Aminomethylation of β,β-Disubstituted Enesulfinamides: Asymmetric Construction of Less Accessible Acyclic α,α-Disubstituted α-Aminomethylated Ketimines
β,β-Disubstituted enesulfinamides undergo stereoselective nucleophilic addition to the formaldehyde imines, in situ formed from tosylmethylcarbamates, affording α-aminomethylated ketimines bearing a challenging acyclic quaternary stereocenter substituted by two sterically and electronically similar groups (e.g., Me and Et). The defined geometry of the C═C bond in the enesulfinamides, combined with the strong chiral induction offered by their chiral sulfinyl group, ensures precise stereocontrol during the formation of the new C–C bond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


