无丝氨酸/甘氨酸饮食在增强抗肿瘤免疫力和通过 PD-L1 乳化促进规避方面的双重影响
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
无丝氨酸/甘氨酸饮食(-SG 饮食)对结直肠癌(CRC)的影响仍不清楚;同时,程序性死亡-1(PD-1)抑制剂对大多数 CRC 患者的疗效较差。在这里,我们证明了 -SG 饮食能抑制 CRC 的生长,并促进细胞毒性 T 细胞的积累,从而增强抗肿瘤免疫力。此外,我们还发现肿瘤细胞中程序性死亡配体1(PD-L1)的乳化是细胞毒性T细胞介导的抗肿瘤反应中的免疫逃避机制,而阻断PD-1/PD-L1信号通路能够恢复-SG饮食所招募的CD8+ T细胞的功能,这表明-SG饮食与免疫疗法的结合具有潜力。我们进行了一项单臂 I 期研究(ChiCTR2300067929)。主要结果表明,-SG 饮食在调节全身免疫力方面是可行且安全的。次要结果包括患者的耐受性和潜在的抗肿瘤效果。总之,我们的研究结果凸显了 -SG 饮食治疗实体瘤的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
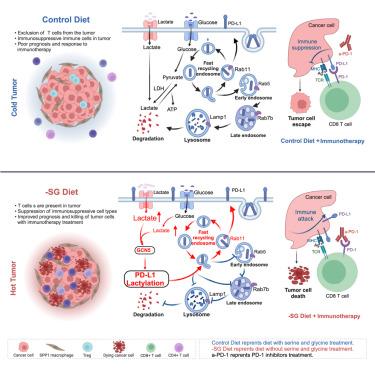
Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation
The effect of the serine/glycine-free diet (−SG diet) on colorectal cancer (CRC) remains unclear; meanwhile, programmed death-1 (PD-1) inhibitors are less effective for most CRC patients. Here, we demonstrate that the −SG diet inhibits CRC growth and promotes the accumulation of cytotoxic T cells to enhance antitumor immunity. Additionally, we also identified the lactylation of programmed death-ligand 1 (PD-L1) in tumor cells as a mechanism of immune evasion during cytotoxic T cell-mediated antitumor responses, and blocking the PD-1/PD-L1 signaling pathway is able to rejuvenate the function of CD8+ T cells recruited by the −SG diet, indicating the potential of combining the −SG diet with immunotherapy. We conducted a single-arm, phase I study (ChiCTR2300067929). The primary outcome suggests that the −SG diet is feasible and safe for regulating systemic immunity. Secondary outcomes include patient tolerability and potential antitumor effects. Collectively, our findings highlight the promising therapeutic potential of the −SG diet for treating solid tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


