共沉淀强化多肽催化作用
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
现在,Ana S. Pina 及其同事报告说,LLPS 可以显著提高多肽的催化活性。具体来说,研究人员创建了基于单肽的凝聚剂,空间限制使肽的结构更加折叠(如图)。肽 P7 (KVYFSIPWRVPM-NH2)被用作模型系统,它对磷酸化集合体有很高的亲和力,能水解磷酸酯分子,并含有公认有利于相分离的精氨酸、赖氨酸、丝氨酸和脯氨酸残基。通过筛选肽和盐的浓度以及不同的温度,找到了诱导形成 P7 共凝胶的合适条件,并进一步优化了实验设计,减少了不必要的反应,如肽的聚集和沉淀以及共凝胶的分裂。然后使用光谱方法研究 LLPS 对肽构象的影响。结果表明,通过 LLPS 的分隔作用,P7 的二级结构趋于稳定。具体来说,与溶液中灵活的发夹样肽相比,检测到了完全折叠的β-发夹结构。此外,肽的电荷和疏水性也会影响共凝胶对客体分子的分配。研究进一步表明,磷酸化的影响更为明显,与未磷酸化的蛋白质分子相比,磷酸化会优先吸收客体分子。随后,以对硝基苯磷酸酯(pNPP)为底物,对凝聚态玻璃进行了磷酸酯水解催化测试,确定了 kcat = (4.9 ± 0.6) × 10-3 s-1 和 KM = (8.2 ± 3.2) × 10-4 M 的催化参数,以及 kcat/KM = 5.9 ± 0.2 的催化效率。令人印象深刻的是,这比之前工作中测定的游离肽在溶液中介导的反应的催化效率提高了 15,000 倍。简单的小肽可以实现自凝聚、选择性招募底物并加速催化,这一点很可能会引起生命起源研究人员的兴趣,也可能会对未来药物输送和传感系统的设计产生启发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
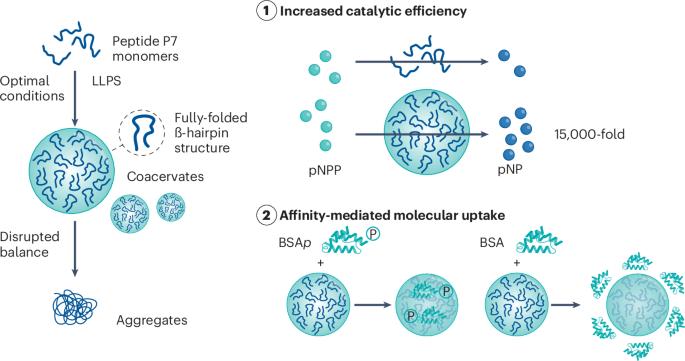
Coacervation-enhanced peptide catalysis
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


