贵金属醋酸盐电化学氧化的机理研究
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
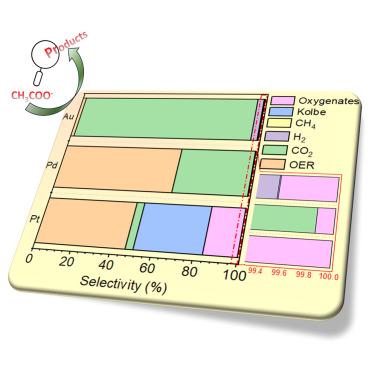
电化学醋酸盐氧化(AcOR)为生产可再生生物燃料提供了一种可持续的方法。从热力学角度来看,CO₂ 的形成是有利的,但醋酸氧化也可通过 Kolbe 和 Hofer-Moest 机制产生各种产物,从而调节通过部分氧化形成的产物。鉴于反应的复杂性,了解不同的反应条件如何影响产物概况至关重要。此外,这一过程还会产生甲基自由基,为甲烷的部分氧化提供启示。目前的研究探讨了贵金属电极(铂、钯、金)在 0.5 M CH3COOK 水电解质中的 AcOR,利用电位和时间依赖性电解及同位素标记实验揭示了产物形成的机理。研究分析了表面化学、离子传输、电解质浓度和电解技术对产物选择性的影响。此外,该研究还将电解槽的产物曲线与批次槽设置中从模型电极获得的曲线进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic insights into the electrochemical oxidation of acetate at noble metals
Electrochemical acetate oxidation (AcOR) offers a sustainable approach to produce renewable biofuels. While CO₂ formation is thermodynamically favored, acetate oxidation can also yield various products through the Kolbe and Hofer-Moest mechanisms, enabling a modulation of the products formed via partial oxidation. Given the complexity of the reaction, it is crucial to understand how different reaction conditions influence the product profile. Furthermore, this process generates methyl radicals, providing insights into methane partial oxidation. The current study explores AcOR on noble metal electrodes (Pt, Pd, Au) in a 0.5 M CH3COOK aqueous electrolyte, revealing the mechanism of product formation using potential- and time-dependent electrolysis and isotope-labeling experiments. The effect of surface chemistry, ion transport, electrolyte concentration, and electrolysis techniques on product selectivity is analyzed. Additionally, the study compares product profiles from an electrolyzer cell to those obtained from model electrodes in batch-cell setup.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


